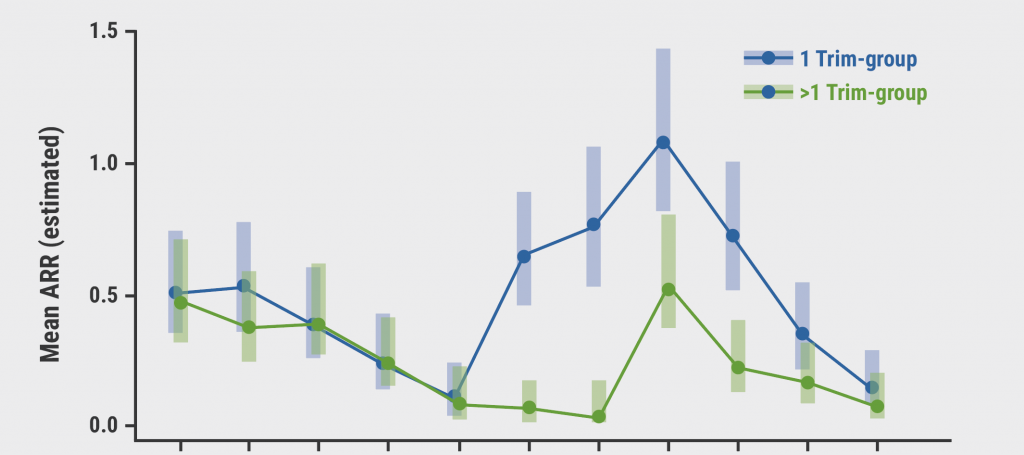
A total of 72 pregnancies in 63 relapsing MS patients were evaluated. Median age was 31.4 years, median disease duration 7.1 years, median EDSS 1.5 at last visit before birth, and median follow-up 6 years. Of 433 included samples, 167 were taken before pregnancy, 92 during pregnancy/DMT-free postpartum, and 174 after pregnancy. Most patients (n=39) were treated with fingolimod or natalizumab as last medication before birth; 4 patients did not use a DMT before, during, and after pregnancy. In 14 of 72 pregnancies, DMT exposure during pregnancy was >6 months; in 39 pregnancies, exposure was limited to the first 2 trimesters; in 15 pregnancies, treatment was discontinued before getting pregnant.
Relapses were more likely in the first trimester and the first 3 months postpartum. sNfL levels increased towards the last trimester and in the first 3 months postpartum, not only in women who relapsed. Mean sNfL levels were 22% higher during pregnancy (β=1.22; P<0.001). During the postpartum, 29 relapses occurred. Relapses were associated with 98% higher sNfL (β=1.98; P<0.001). Mean sNfL was 13% higher during the postpartum period (β=1.13; P=0.009). However, this difference lost significance after including DMT exposure into the model (β=1.07; P=0.178). Patients sampled during DMT had on average 12% lower sNfL levels compared with patients without (β=0.88; P=0.019). The authors concluded that strategies allowing to continue DMT during pregnancy may be warranted.
- Yaldizli Ö, et al. Interrupting disease modifying treatment for pregnancy in multiple sclerosis – effect on disease activity and serum neurofilament light chain. MSVirtual 2020, Abstract LB01.06.
Posted on
Previous Article
« Predicting autoimmunity in patients treated with alemtuzumab Next Article
Serum NfL as biomarker for suboptimal treatment response »
« Predicting autoimmunity in patients treated with alemtuzumab Next Article
Serum NfL as biomarker for suboptimal treatment response »
Table of Contents: MS Virtual 2020
Featured articles
Online First
Positive results for vagus nerve stimulation in RA
COVID-19 and MS
Biomarkers
Treatment Strategies and Results
Management of progressive MS with approved DMT
Novel Treatment Directions
Positive results for vagus nerve stimulation in RA
Neuromyelitis Optica Spectrum Disorders
Miscellaneous Topics
Related Articles
December 20, 2022
MS activity and pregnancy outcomes after long-term use of natalizumab

© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

