https://doi.org/10.55788/4feffa98
A prospective, observational cohort study assessed relapses during pregnancy and postpartum as well as pregnancy outcomes, including foetal haematological abnormalities, in women with highly-active MS who used natalizumab [1]. Patients were divided into 2 groups: those who terminated natalizumab during the first trimester (1 Trim-group) or after the first trimester (>1 Trim-group). Prof. Sandra Thiel (Ruhr University Bochum, Germany) presented the results [1].
Included were 350 pregnant women, 171 in the 1 Trim-group and 179 in the >1 Trim-group. The latter group stopped natalizumab after a median of 31 gestational weeks (range 12.1–39.7 weeks). Women in the >1 Trim-group had significantly fewer relapses during pregnancy (5.2% vs 32.4%; P<0.001) and postpartum (22.8% vs 49.7%; P<0.001) compared with the 1 Trim-group (see Figure). Early restart (4 weeks postpartum) also decreased the relapse risk postpartum (OR 0.32; 95% CI 0.17–0.58; P<0.001).
Figure: Relapse rate during pregnancy and postpartum [1]
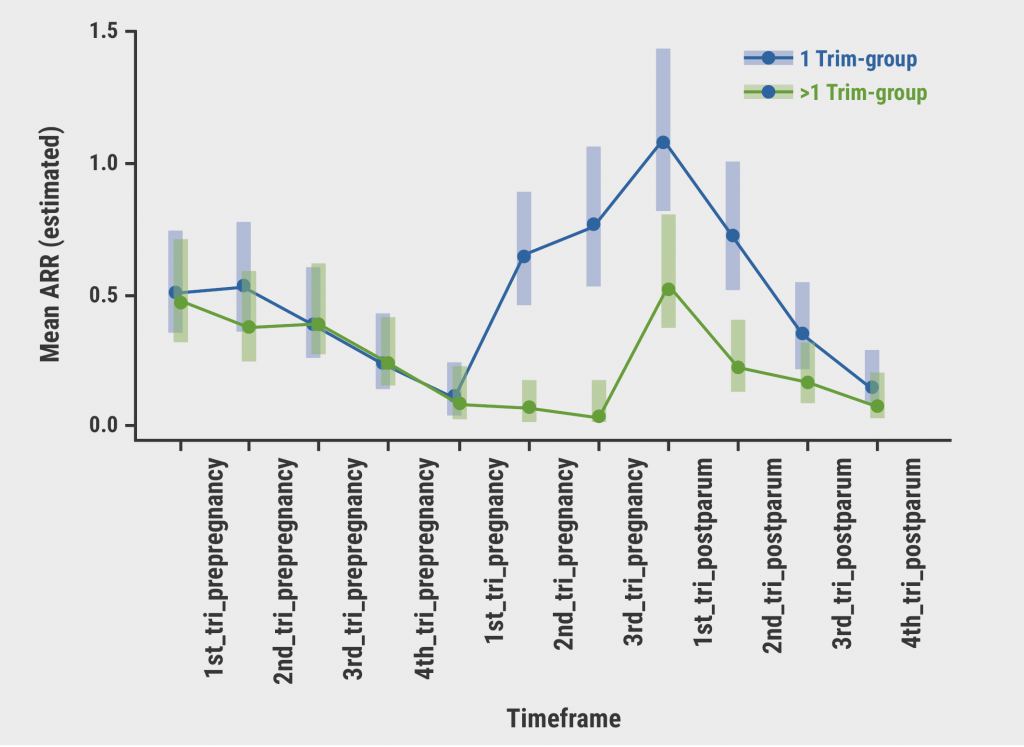
Tri, trimester; 1 Trim-group, natalizumab terminated during the first trimester; >1 Trim-group, natalizumab terminated after the first trimester.
Pregnancy outcomes were similar between groups. In the >1 Trim group and the 1 Trim group, 12.9% and 10.6% of births were preterm (P=0.625), while 4.7% and 3.4% of newborns had congenital abnormalities (P=0.719). In both groups, newborns were smaller than expected. To assess anaemia and thrombocytopenia in the 122 newborns with available blood counts, the >1 Trim group was stratified into women who stopped natalizumab after gestational week 30 (>30GW; n=79) and before week 30 (<30GW; n=43). Compared with the <30GW group, newborns in the >30GW group had significantly more often haematological abnormalities (57% vs 39.5%; P=0.10). Rates of anaemia were 46.8% and 27.9%, for <30GW and >30GW respectively; rates of thrombocytopenia were 22.8% and 16.3%. Significantly more women in the <30GW group suffered from relapse (38.5% vs 16%), especially during the first postpartum trimester.
Prof. Thiel concluded: “These study results should lead to a risk-benefit discussion between women treated with natalizumab and their neurologists, to continue treatment up to week 30 or even week 34 of gestation, in combination with an early restart during the first 4 weeks after delivery.”
- Thiel S. Disease activity and pregnancy outcomes after long-term exposure to natalizumab during pregnancy. Abstract O039, ECTRIMS 2022, 26–28 October, Amsterdam, the Netherlands.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Early treatment with DMT effective in paediatric-onset MS Next Article
New safety data of anti-CD20 mAbs around pregnancy »
« Early treatment with DMT effective in paediatric-onset MS Next Article
New safety data of anti-CD20 mAbs around pregnancy »
Table of Contents: ECTRIMS 2022
Featured articles
Letter from the Editor
Diagnosis and Prediction of Disease Course
A case for including optic nerve lesions in the McDonald criteria
Cerebrospinal fluid kappa-free light chains for MS diagnosis
Early, non-disabling relapses increase disability accumulation
Physical impairment is present before perceived MS onset
Chronic active MS lesions respond poorly to anti-CD20 antibodies
Treatment: Trials & Strategies
Dimethyl fumarate reduces the risk of a first clinical event in RIS
How and when to make a timely switch to high-efficacy DMT
Comparing real-world effectiveness of DMTs
Study fails to show non-inferiority of rituximab to ocrelizumab
Autologous haematopoietic stem cell transplantation versus DMTs
Progressive MS
Stem cell transplantation not superior to natalizumab in progressive MS
Efficacy of DMTs fades away in secondary progressive MS
Smartphone tapping can help detect progressive MS
Paediatric MS
Early treatment with DMT effective in paediatric-onset MS
Fingolimod in paediatric MS: results of up to 6 years
Switching treatment after initial platform injectable DMT: real-world data
Pregnancy
Pregnancy and infant outcomes in women receiving ocrelizumab
New safety data of anti-CD20 mAbs around pregnancy
MS activity and pregnancy outcomes after long-term use of natalizumab
NMOSD
Ravulizumab significantly reduced relapses in AQP4+ NMOSD
NMOSD patients are cognitively impaired regardless of serostatus
Evidence-based consensus on pregnancy in NMOSD
COVID-19
COVID-19 and MS: lessons learned thus far
Ocrelizumab and fingolimod increase the risk of COVID-19 and of worse outcomes
Humoral and cellular immune responses after SARS-CoV-2 vaccination
Miscellaneous
Re-myelination strategies in MS still pose many unanswered questions
MS associated with a broader Epstein-Barr virus specific T-cell receptor repertoire
Cognitive rehab and mindfulness reduce cognitive complaints in MS
Related Articles
December 19, 2022
Chronic active MS lesions respond poorly to anti-CD20 antibodies

August 27, 2019
Prognostic blood and MRI biomarkers
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

