Our current knowledge about the parameters for I-O success has been described in “a wonderful paper which was recently published in Nature Medicine [2]”, Prof. Peters said. “Some intrinsic resistance mechanisms in the tumour are described, which are increasingly being addressed by new compounds.” Furthermore, this knowledge provides a rationale for the evaluation of new I-O/I-O combinations, in which the individual compounds have distinct mechanisms of action.
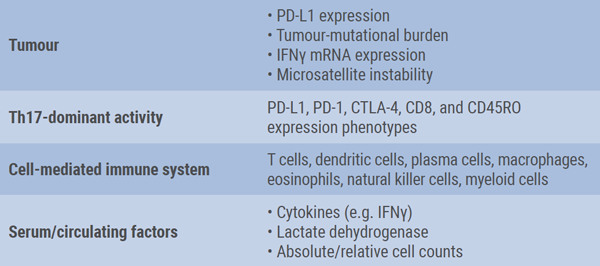
She also addressed the lack of suitable biomarkers for patient selection and monitoring. In the ongoing effort to develop specific biomarkers for ICI therapy, multiple factors should be taken into consideration, i.e. the tumour, tumour microenvironment, and immune system (see Table) [3]. One major issue is the challenge to obtain adequate tumour tissue for molecular testing in patients with advanced NSCLC. So, there is still an unmet medical need for diagnostic approaches that do not require tissue to identify patients who may benefit from immunotherapy. Currently, novel blood-based assays to measure TMB in plasma are being developed [4]. However, a drawback of this method is the presence of intra-tumoral heterogeneity, Prof. Peters mentioned. As a consequence, the set of variants found in a biopsy differs substantially from the DNA fragments released in blood.
Table: Key elements in biomarker development for immune-checkpoint inhibitor therapy [3]
Combination with angiogenesis inhibitors
Finally, according to Prof. Peters, combining angiogenesis inhibitors and I-O also makes sense. Angiogenic factors impair lymphocyte trafficking across endothelia. Vascular endothelial growth factor (VEGF) has profound effects on cancer immunity by inhibiting dendritic cell maturation and antigen presentation, by inhibiting T cell responses, by inducing proliferation of regulatory T cells, and by favouring accumulation of myeloid-derived suppressor cells. The IMpower 150 trial has shown that first-line treatment with carboplatin, paclitaxel, bevacizumab (anti-VEGF), and atezolizumab in patients with advanced non-squamous NSCLC improves PFS [5].
- Galluzzi L, et al. Cancer Immunol Res. 2016;4:895-902.
- Keenan TE, et al. Nat Med. 2019;25:389-402.
- Nishino M, et al. Nat Rev Clin Oncol. 2017;14:655-668.
- Gandara DR, et al. Nat Med. 2018;24:1441-1448.
- Reck M, et al. Ann Oncol. 2017;28(suppl 11):abstract LBA1_PR.
Posted on
Previous Article
« Experiences from France Next Article
Brain irradiation as treatment option »
« Experiences from France Next Article
Brain irradiation as treatment option »
Table of Contents: ELCC 2019
Featured articles
Electromagnetic navigation bronchoscopy
Current Management of Early Stage NSCLC
Trial Data: Early Stage Lung Cancer
Electromagnetic navigation bronchoscopy
Genomic and immune profiling
Immunotherapy in Stage 4 Lung Cancer
Other I-O combinations
Predictive diagnostics for I-O
Trials: Immunotherapy in Stage 4 Lung Cancer
Post-study immunotherapy in MYSTIC
Implementation of Personalised Lung Cancer Care in Clinical Routine
How can societies help to implement personalised treatment?
Optimal Management of Brain Metastases in NSCLC
Incidence and local treatment
Brain irradiation as treatment option
Small Cell Lung Cancer: New Targets
Molecular characteristics of SCLC
Immunotherapy in SCLC: trial data
Related Articles

June 25, 2019
Letter from the editor
June 25, 2019
Brain irradiation as treatment option
June 25, 2019
Electromagnetic navigation bronchoscopy
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

