In a previous analysis, presented at the 2018 ESMO I-O meeting, an improvement in OS was seen with durvalumab vs chemotherapy in patients with tumour cell PD-L1 expression ≥25% (see Figure; HR 0.76, P=0.036) [3]. The safety and tolerability profile of durvalumab was consistent with data from previous trials [1,2,4].
Figure: OS in patients with PD-L1 expression of ≥25%: primary endpoint of MYSTIC trial [3]
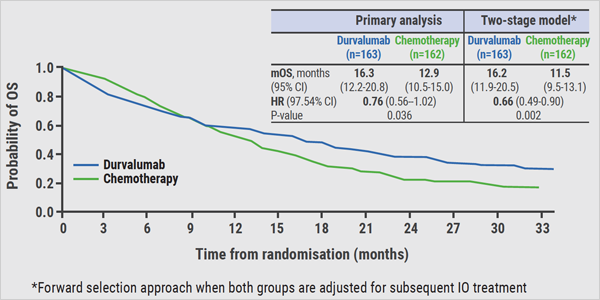
Figure kindly provided by Dr Reinmuth.
In the current analysis of MYSTIC, Dr Niels Reinmuth (Asklepios Lung Clinic, Munich-Gauting, Germany) described subsequent treatment patterns and explored the effect of subsequent immunotherapy on the OS outcome with durvalumab vs chemotherapy [5]. Among patients who received subsequent treatment, immunotherapy was administered to 14% of patients in the durvalumab group and 67% of patients in the chemotherapy group, so a markedly higher proportion of patients. This imbalance with respect to the subsequent policy may have confounded the primary OS outcome. The current exploratory analysis showed increased OS benefit with first-line durvalumab vs chemotherapy after adjusting for the effect of subsequent immunotherapy (see Figure; HR 0.66, P=0.002) [5]. More long-term data about the impact of immunotherapy on OS in this setting is needed.
1. Garassino MC, et al. Lancet Oncol. 2018;19:521-536.
2. Kowalski DM, et al. Ann Oncol. 2018;29(suppl_8):viii493-viii547.
3. Rizvi N, et al. Ann Oncol. 2018;29(suppl 10). Presented at ESMO I-O 2018, abstract LBA6.
4. Antonia SJ, et al. N Engl J Med. 2017;377:1919-1929.
5. Reinmuth N, et al. ELCC 2019, abstract LBA4.
Posted on
Previous Article
« Predictive diagnostics for I-O Next Article
Letter from the editor »
« Predictive diagnostics for I-O Next Article
Letter from the editor »
Table of Contents: ELCC 2019
Featured articles
Electromagnetic navigation bronchoscopy
Current Management of Early Stage NSCLC
Trial Data: Early Stage Lung Cancer
Electromagnetic navigation bronchoscopy
Genomic and immune profiling
Immunotherapy in Stage 4 Lung Cancer
Other I-O combinations
Predictive diagnostics for I-O
Trials: Immunotherapy in Stage 4 Lung Cancer
Post-study immunotherapy in MYSTIC
Implementation of Personalised Lung Cancer Care in Clinical Routine
How can societies help to implement personalised treatment?
Optimal Management of Brain Metastases in NSCLC
Incidence and local treatment
Brain irradiation as treatment option
Small Cell Lung Cancer: New Targets
Molecular characteristics of SCLC
Immunotherapy in SCLC: trial data
Related Articles
September 13, 2021
Lung-cancer trial enrollment takes a hit amid COVID-19

© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

