Dr Luis Paz-Ares (Complutense University of Madrid, Spain) gave an overview about the combination of I-O and chemotherapy. The data of previous phase 1 studies evaluating front-line therapy of anti-PD-L1 or anti-PD1 agents in combination with chemotherapy were “not overwhelming”, Dr Paz-Ares mentioned [3]. “The response rates were in the range of 50%.” However, further research showed some more positive results. For example, the open-label Keynote-021 suggests that combination of pembrolizumab, carboplatin, and pemetrexed could be an effective and tolerable first-line treatment option for patients with advanced non-squamous NSCLC [4].
Subsequently, the Keynote-189 trial showed that in patients with previously untreated, metastatic, non-squamous NSCLC without EGFR or ALK mutations, the addition of pembrolizumab to standard chemotherapy resulted in significantly longer OS and PFS than chemotherapy alone (OS at 12 months: 69.2% vs 49.4%; HR for death 0.49; P<0.001). Improvement in OS was seen across all PD-L1 categories evaluated [5]. Comparable results were found in the Impower-132 trial, evaluating first-line combination of atezolizumab, carboplatin/cisplatin, and pemetrexed in stage 4 non-squamous NSCLC [6].
Potential implications
Dr Paz-Ares also provided insight into his current treatment decisions and possible policy in the future. “In most patients with high PD-L1 expression, I currently use immunotherapy. For patients with aggressive disease, with a high need for a quick symptomatic improvement, I would recommend chemo/I-O. For patients with moderate or low PD-L1 expression, chemo/I-O is preferred. Maybe in the future, but that is merely speculation, we are going to select patients based on TMB (see Table, his preferred options are shown in red).”
Table: Speculated treatment overview for patients selected on TMB
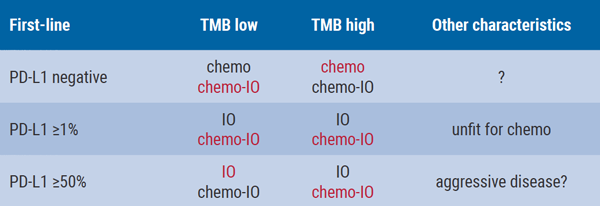
Options in red are preferred by Dr Paz-Ares.
- Emens LA, Middleton G. Cancer Immunol Res. 2015;3:436-43.
- Galluzzi L, et al. Cancer Immunol Res. 2016;4:895-902.
- Giaccone G, et al. Eur J Cancer 2015; 51(Suppl. 3): S107–S108.
- Langer CJ, et al. Lancet Oncol. 2016;17:1497-1508.
- Gandhi L, et al. N Engl J Med. 2018;378:2078-2092.
- Papadimitrakopoulou VA, et al. WCLC 2018, abstract #OA05.07.
Posted on
Previous Article
« Brain irradiation as treatment option Next Article
Tumour, node, and metastasis (TNM) staging system »
« Brain irradiation as treatment option Next Article
Tumour, node, and metastasis (TNM) staging system »
Table of Contents: ELCC 2019
Featured articles
Electromagnetic navigation bronchoscopy
Current Management of Early Stage NSCLC
Trial Data: Early Stage Lung Cancer
Electromagnetic navigation bronchoscopy
Genomic and immune profiling
Immunotherapy in Stage 4 Lung Cancer
Other I-O combinations
Predictive diagnostics for I-O
Trials: Immunotherapy in Stage 4 Lung Cancer
Post-study immunotherapy in MYSTIC
Implementation of Personalised Lung Cancer Care in Clinical Routine
How can societies help to implement personalised treatment?
Optimal Management of Brain Metastases in NSCLC
Incidence and local treatment
Brain irradiation as treatment option
Small Cell Lung Cancer: New Targets
Molecular characteristics of SCLC
Immunotherapy in SCLC: trial data
Related Articles

June 25, 2019
Letter from the editor
June 25, 2019
Predictive diagnostics for I-O
June 25, 2019
Tumour, node, and metastasis (TNM) staging system
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

