TERIKIDS (NCT02201108) is a 2-year, multicentre, multinational, randomised, double-blind, placebo-controlled, parallel-group phase 3 study of teriflunomide in children and adolescents of 10-17 years at baseline with relapsing MS. It is followed by a 96-week open-label period, of which interim results were shared. All participants in the open-label period (n=152) received teriflunomide at a dose based on body weight, equivalent to 14 mg in adults.
The primary endpoint in the double-blind period, median time to first confirmed relapse, was 75.3 weeks with teriflunomide and 39.1 weeks with placebo (HR 0.66; 95% CI 0.39–1.1). This difference was not statistically significant versus placebo (P=0.29). In a prespecified sensitivity analysis, median time to first disease activity was significantly reduced by teriflunomide (HR 0.57; 95% CI 0.37-0.87; P=0.041).
Differences in key secondary outcomes were also significant. There was relative reduction of 75% in the number of T1 gadolinium-enhancing lesions versus placebo (P<0.0001), and a relative reduction of 55% in the number of new/enlarging T2 lesions (P=0.0006). Median time to first confirmed relapse in the double-blind and open-label period combined was numerically lower with continuous teriflunomide versus placebo/teriflunomide (HR 0.61), as was time to disability progression (HR 0.552; see Figure). The number of new/enlarging T2 lesions per MRI scan was reduced with continuous teriflunomide versus placebo/teriflunomide (6.3 vs 13.0; P=0.0006). The number of T1 gadolinium-enhancing lesions was 4.2 versus 1.9 (P=0.0106).
Incidence of adverse events during the open-label period was lower with continuous teriflunomide than with placebo/teriflunomide: 68.0% versus 82.7%. In 8 patients, adverse events (2 serious) led to treatment discontinuation during the open-label period.
Figure: Time to disability progression sustained for 24 weeks [1]
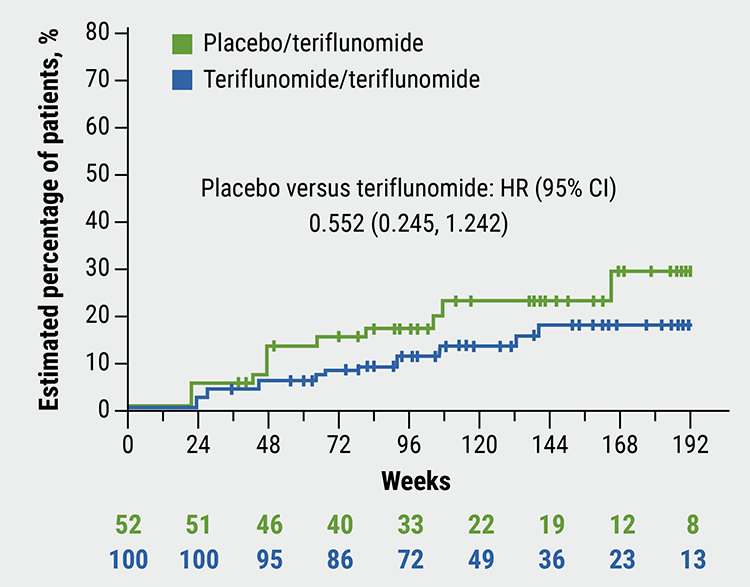
- Chitnis T, et al. Teriflunomide efficacy and safety in pediatric patients with relapsing forms of MS: Interim analysis of open-label TERIKIDS trial extension. MSVirtual 2020, Abstract FC02.04.
Posted on
Previous Article
« Teriflunomide improves satisfaction versus prior DMT regardless of age Next Article
Low-dose rituximab as effective as high-dose, but safer »
« Teriflunomide improves satisfaction versus prior DMT regardless of age Next Article
Low-dose rituximab as effective as high-dose, but safer »
Table of Contents: MS Virtual 2020
Featured articles
Online First
Positive results for vagus nerve stimulation in RA
COVID-19 and MS
Biomarkers
Treatment Strategies and Results
Management of progressive MS with approved DMT
Novel Treatment Directions
Positive results for vagus nerve stimulation in RA
Neuromyelitis Optica Spectrum Disorders
Miscellaneous Topics
Related Articles
November 25, 2020
Update on estimated PML risk related to fingolimod
August 27, 2019
Autologous haematopoietic stem cell transplantation

August 27, 2019
Prognostic blood and MRI biomarkers
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

