The study cohort (COVISEP registry) that was analysed included 405 MS patients with confirmed or highly suspected SARS-CoV-2 infection between 1 March 2020 and 14 July 2020. Included were patients who met at least one of the following criteria:
- biologically confirmed COVID-19 diagnosis based on SARS-CoV-2 RT-PCR positivity;
- typical thoracic CT abnormalities (ground-glass opacities) in epidemic areas;
- sudden-onset anosmia or ageusia in the absence of rhinitis or nasal obstruction; or
- typical symptoms (triad associating cough, fever, asthenia) in the epidemic zone of COVID-19.
Mean age was 44.7 years, mean MS duration was 13.4 years, and 293 patients (72%) were female. Median EDSS was 2.0 (range 0.0-9.5) and 326 patients (80.5%) used a disease-modifying treatment (DMT). COVID-19 severity was assessed on a 7-point ordinal scale, ranging from 1 (not hospitalised, no limitations on activities) to 7 (death). Cut-off score was at 3 (hospitalised, not requiring supplemental oxygen). The presented results were a follow-up on previously published results in JAMA Neurology [2].
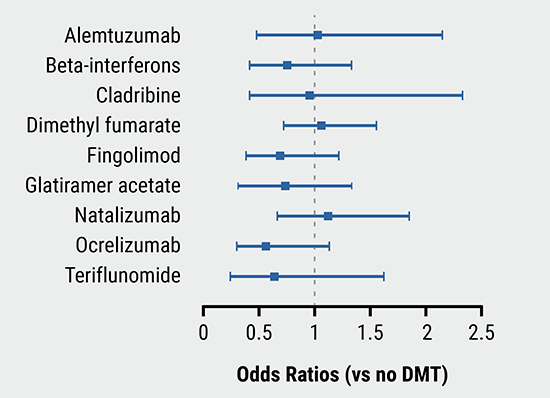
Of 405 participants, 78 (19.3%) had a COVID-19 severity score ≥3 and 12 patients (3.0%) died from COVID-19. Most of the very severe COVID-19 patients did not use any DMT. The percentage of patients with a COVID-19 severity score ≥3 in patients with and without a DMT was 14.4% versus 39.2% (P<0.001). Independent risk factors for COVID-19 severity score ≥3 were higher age (OR 1.8 for 10 years) and higher EDSS (OR 4.5 for EDSS ≥6). Obesity and cardiac comorbidity were also associated with severe COVID-19 (OR 2.58 and 2.39, respectively). Immunomodulatory treatment with interferon or glatiramer acetate was associated with a lower risk of COVID-19 severity score ≥3 (OR 0.2) compared with no treatment. Knowing these risk factors should help to guide individualised clinical management of MS patients during the COVID-19 pandemic.
- Louapre C, et al. Clinical Characteristics and Outcomes in Patients with Coronavirus Disease 2019 and Multiple Sclerosis. MSVirtual 2020, Abstract SS02.06
- Louapre C, et al. JAMA Neurol. 2020;77(9):1079-88.
Posted on
Previous Article
« Anti-CD20 DMTs associated with worse COVID-19 outcomes Next Article
Risk of COVID-19 not increased in MS patients »
« Anti-CD20 DMTs associated with worse COVID-19 outcomes Next Article
Risk of COVID-19 not increased in MS patients »
Table of Contents: MS Virtual 2020
Featured articles
Online First
Positive results for vagus nerve stimulation in RA
COVID-19 and MS
Biomarkers
Treatment Strategies and Results
Management of progressive MS with approved DMT
Novel Treatment Directions
Positive results for vagus nerve stimulation in RA
Neuromyelitis Optica Spectrum Disorders
Miscellaneous Topics
Related Articles

December 20, 2022
Humoral and cellular immune responses after SARS-CoV-2 vaccination
November 25, 2020
Risk of COVID-19 not increased in MS patients
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

