Dr Alan Klein (Cleveland Clinic, USA) presented the main results of the RHAPSODY trial (NCT03737110). As Dr Klein explained, recurrent pericarditis is a debilitating disease with no (FDA) approved therapies; typically a combination of non-steroidal anti-inflammatory drugs (NSAIDs), colchicine, and steroids is given. In the long run, steroids are associated with significant morbidity. IL-1 has been implicated as a key mediator in recurrent pericarditis. Rilonacept is a subcutaneous, once-weekly IL-1α and IL-1β cytokine trap.
RHAPSODY was a phase 3, multicentre, double-blind, event-driven, randomised withdrawal trial of rilonacept. Acutely symptomatic patients failing their background regimen (n=86) received rilonacept 320 mg, followed by a run-in period with rilonacept 160 mg weekly for 12 weeks (during which background medication was tapered), after which clinical responders were randomised to rilonacept 160 mg weekly monotherapy (n=30) versus placebo (n=31). The primary efficacy endpoint was time to first adjudicated pericarditis recurrence. Dr Klein stressed that enrolled patients were representative of the real-world pericarditis population.
Rilonacept initiation resulted in rapid resolution of acute pericarditis episodes. Pain numerical rating scale and C-reactive protein (CRP) rapidly decreased after the first dose of rilonacept. All patients on corticosteroids successfully tapered and transitioned to monotherapy rilonacept during the run-in. The primary outcome occurred in 2 of 30 (6.7%) of patients in the rilonacept group and in 23 of 31 (74.2%) in the placebo group (P<0.0001). Median number of weeks to recurrence in the rilonacept group could not be calculated due to the number of events being too low; in the placebo group it was 8.6 weeks (95% CI 4.0–11.7); HR 0.04 (95% CI 0.01–0.18; P<0.001; see Figure). The 2 events in the rilonacept arm both occurred after brief, temporary drug interruptions.
Figure: Rilonacept significantly reduced risk of pericarditis recurrence [1]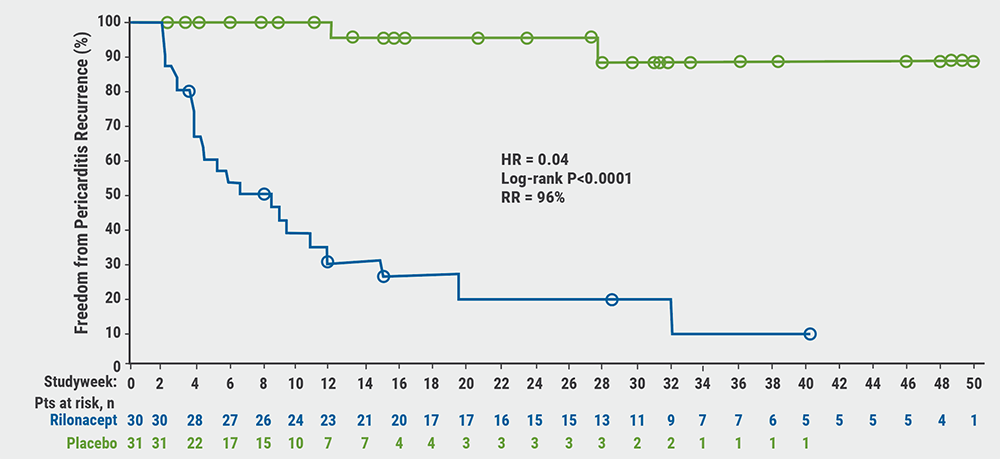
The rates of 3 secondary efficacy endpoints were statistically highly significantly in favour of rilonacept:
- the proportion of patients that maintained clinical response at week 16 was 81% in the rilonacept group versus 20% in the placebo group (P=0.0002);
- the proportion of patients with absent/minimal pericarditis symptoms (based on the 6-point Patient Global Impression of Pericarditis Severity [PGIPS] at week 16) was 81% and 25%, respectively (P=0.0006); and
- the percentage of days with no/minimal pain in the first 16 weeks was 98% and 46%, respectively (P=0.0001).
Rilonacept was generally well-tolerated with no drug-related serious adverse events or deaths. In the run-in period, 4 patients discontinued rilonacept therapy due to adverse events. The most common adverse events with rilonacept were injection-site reactions and upper respiratory tract infections.
- Klein AL. RHAPSODY: Rilonacept an IL-1α and IL-1β Trap Resolves Pericarditis Episodes and Reduces Risk of Recurrence in a Phase 3 Trial of Patients With Recurrent Pericarditis. LBS.07, AHA Scientific Sessions 2020, 13–17 Nov.
- Klein AL, et al. N Engl J Med 2021;384:31–41.
Posted on
Previous Article
« Intensive blood pressure lowering benefits older adults Next Article
Transgender people have unaddressed heart disease risks »
« Intensive blood pressure lowering benefits older adults Next Article
Transgender people have unaddressed heart disease risks »
Table of Contents: AHA 2020
Featured articles
COVID-19 and Influenza
Fewer CV complications than expected in AHA COVID-19 Registry
Worse COVID-19 outcomes in younger obese patients
Effects of CVD in hospitalised COVID-19 patients
Unfavourable outcomes for COVID-19 patients with AF and atrial flutter
High-dose influenza vaccine in patients with CVD
Atrial Fibrillation
Vitamin D or omega 3 fatty acids do not prevent AF
Active screening for AF improves clinical outcomes
AF screening in older adults at primary care visits
CVD Risk Reduction
Clever trial design gets patients back on statins: the SAMSON trial
Polypill plus aspirin reduces cardiovascular events
Lowering LDL cholesterol in older patients is beneficial
No CV benefit from omega 3 in high-risk patients
Safety and efficacy of inclisiran for hypercholesterolemia
Remote risk management programme effective and efficient
Healthy lifestyle lowers mortality irrespective of medication burden
Heart Failure
Omecamtiv mecarbil improves outcomes in HFrEF-patients
IV iron reduces HF hospitalisation
Dapagliflozin reduces renal risk independent of CV disease status
“Strongly consider an SGLT2-inhibitor in most T2DM patients”
Additional HFrEF education and patient-engagement tools
Acute Coronary Syndrome
No benefit from omega-3 fatty acids after recent MI
PIONEER III trial: Drug-eluting stents comparable
Coronary and Valve Disease
Extra imaging reveals cause of MINOCA in women
Ticagrelor not superior to clopidogrel after elective PCI
Stroke
Ticagrelor/aspirin reduces stroke risk in patients with ipsilateral cervicocranial plaque
AF monitoring following cardiovascular surgery
Miscellaneous
PAD: Rivaroxaban reduces VTE risk after revascularisation
Sotatercept: potential new treatment option for PAH
Finerenone lowers CV events in diabetic CKD patients
Mavacamten effective in obstructive hypertrophic cardiomyopathy
Children exposed to tobacco smoke have worse heart function as adults
Transgender people have unaddressed heart disease risks
Intensive blood pressure lowering benefits older adults
Longer chest compression pause worsens outcomes after paediatric IHCA
Related Articles
February 17, 2021
High-dose influenza vaccine in patients with CVD
February 17, 2021
Worse COVID-19 outcomes in younger obese patients

February 24, 2021
Letter from the Editor
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

