The TIPS-3 study (NCT01646437) is a randomised controlled trial with a 2x2x2 factorial design, conducted in 9, mainly South Asian countries. Prof. Salim Yusuf (Population Health Research Institute, Canada) presented the results, which were simultaneously published in the New England Journal of Medicine [1,2]. TIPS-3 randomised 5,713 people who did not have CVD but were classified as being at intermediate or high risk as measured by the INTERHEART Risk Score. Mean age of the participants was 64 years, and 53% were female. The polypill contained atenolol (100 mg), ramipril (10 mg), hydrochlorothiazide (25 mg), and simvastatin (40 mg). In a 2-by-2-by-2 factorial design, one arm of the TIPS-3 trial tested the polypill versus placebo, another arm tested the polypill in combination with aspirin (75 mg daily) versus double placebo, and a third arm tested aspirin versus placebo. Each intervention included a control group that received a matching placebo. The primary outcome was a composite of death from cardiovascular causes and heart failure, resuscitation from cardiac arrest, or arterial revascularisation.
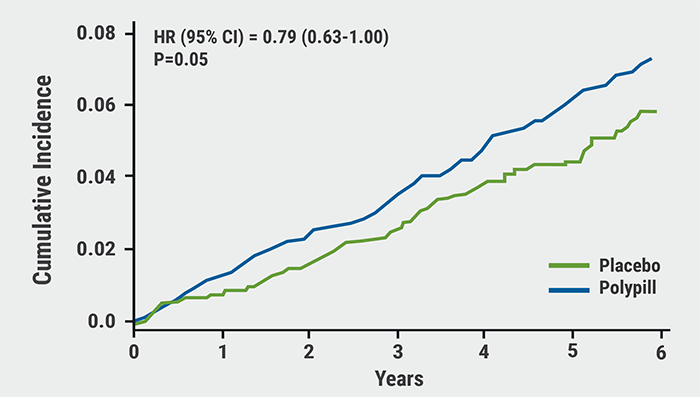
After a mean follow-up of 4.6 years, the primary outcome was reached by 4.4% in the polypill group (n=2,861) compared with 5.5% in its placebo group (n=2,852): a difference of 21% (HR 0.79; 95% CI 0.63–1.00; P=0.05). Adding aspirin to the polypill resulted in an even greater treatment benefit versus placebo: events occurred in 4.1% of the polypill + aspirin group (n=1,429) versus 5.8% in the matching double-placebo group (n=1,421): a difference of 31% (HR 0.69; 95% CI 0.50–0.97; P=0.03) (see Figure). A sensitivity analysis showed that this benefit was larger in those who were adherent to treatment (HR 0.61; 95% CI 0.41–0.91). The primary outcome occurred in 4.1% in the aspirin group (n=2,860) versus 4.7% in its placebo group (n=2,853): a difference of 14% (HR 0.86; 95% CI 0.67–1.10). All treatments had good safety profiles, although hypotension and dizziness were more common in the group taking the polypill and aspirin.
Figure: Polypill + aspirin versus double placebo — primary outcome. Modified from [2]
Reflecting on the clinical implications of the results, Prof. Yusuf highlighted the following:
- A CVD risk reduction of 30-40% with polypill + aspirin is less than was originally hypothesised (likely due to multiple challenges the trial faced, including a high incidence of discontinuation due to reasons unrelated to side effects and site-restrictions due to the COVID-19 pandemic), but is nevertheless important.
- If half of eligible people used a polypill with aspirin, 3 to 5 million CVD events could be avoided worldwide each year.
- It is likely a cost-effective strategy to meet the global targets of reducing CVD by 30% by 2030.
- Future polypills that could reduce low-density lipoprotein cholesterol and blood pressure to a greater extent might lead to larger benefits.
- Yusuf S, et al. Aspirin Alone and in Combination With a Polypill in Cardiovascular Disease Primary Prevention: Results From the International Polycap Study (TIPS)-3. LBS.02, AHA Scientific Sessions 2020, 13–17 Nov.
- Yusuf S et al. New Engl J Med, Nov 13, 2020.DOI: 10.1056/NEJMoa2028220.
Posted on
Previous Article
« Lowering LDL cholesterol in older patients is beneficial Next Article
Clever trial design gets patients back on statins: the SAMSON trial »
« Lowering LDL cholesterol in older patients is beneficial Next Article
Clever trial design gets patients back on statins: the SAMSON trial »
Table of Contents: AHA 2020
Featured articles
COVID-19 and Influenza
Fewer CV complications than expected in AHA COVID-19 Registry
Worse COVID-19 outcomes in younger obese patients
Effects of CVD in hospitalised COVID-19 patients
Unfavourable outcomes for COVID-19 patients with AF and atrial flutter
High-dose influenza vaccine in patients with CVD
Atrial Fibrillation
Vitamin D or omega 3 fatty acids do not prevent AF
Active screening for AF improves clinical outcomes
AF screening in older adults at primary care visits
CVD Risk Reduction
Clever trial design gets patients back on statins: the SAMSON trial
Polypill plus aspirin reduces cardiovascular events
Lowering LDL cholesterol in older patients is beneficial
No CV benefit from omega 3 in high-risk patients
Safety and efficacy of inclisiran for hypercholesterolemia
Remote risk management programme effective and efficient
Healthy lifestyle lowers mortality irrespective of medication burden
Heart Failure
Omecamtiv mecarbil improves outcomes in HFrEF-patients
IV iron reduces HF hospitalisation
Dapagliflozin reduces renal risk independent of CV disease status
“Strongly consider an SGLT2-inhibitor in most T2DM patients”
Additional HFrEF education and patient-engagement tools
Acute Coronary Syndrome
No benefit from omega-3 fatty acids after recent MI
PIONEER III trial: Drug-eluting stents comparable
Coronary and Valve Disease
Extra imaging reveals cause of MINOCA in women
Ticagrelor not superior to clopidogrel after elective PCI
Stroke
Ticagrelor/aspirin reduces stroke risk in patients with ipsilateral cervicocranial plaque
AF monitoring following cardiovascular surgery
Miscellaneous
PAD: Rivaroxaban reduces VTE risk after revascularisation
Sotatercept: potential new treatment option for PAH
Finerenone lowers CV events in diabetic CKD patients
Mavacamten effective in obstructive hypertrophic cardiomyopathy
Children exposed to tobacco smoke have worse heart function as adults
Transgender people have unaddressed heart disease risks
Intensive blood pressure lowering benefits older adults
Longer chest compression pause worsens outcomes after paediatric IHCA
Related Articles
February 18, 2021
No benefit from omega-3 fatty acids after recent MI

February 17, 2021
Polypill plus aspirin reduces cardiovascular events
February 18, 2021
Transgender people have unaddressed heart disease risks
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

