Prof. Dean Wingerchuk (Mayo Clinic Phoenix/Scottsdale, AZ, USA) explained that given the absence of any head-to-head studies, his team conducted an indirect comparison based on published studies up to September 2020 from controlled trials testing eculizumab, satralizumab, and inebilizumab individually. He also acknowledged that there are limitations to this type of analysis, and pointed out that all 3 agents are very effective in treating AQP4+ NMOSD.
The meta-analysis incorporated data from 29 studies from 4 placebo-controlled clinical trials — collectively including 551 patients: N-MOmentum phase 2/3 clinical trial (NCT02200770), PREVENT phase 3 trial (NCT01892345), and SAkuraStar (NCT02073279) and SAkuraSky (NCT02028884) phase 3 trials. Time-to-first relapse was the only outcome measure reported across all trials and was therefore used in the comparison efficacy analysis.
The researchers conducted 3 separate analyses: 1 comparing the 3 therapies alone, another comparing eculizumab with satralizumab, given alone or in combination with ISTs, and the 3rd comparing the eculizumab-IST combination with the satralizumab-IST combination. The results showed that eculizumab alone led to a significantly reduced risk of first relapse, compared with both satralizumab (by 90%) and inebilizumab (by 89%). When given alone or in combination with ISTs, eculizumab was also associated with a 76% reduced risk of first relapse relative to satralizumab. Furthermore, patients treated with combination eculizumab-IST therapy were 59% less likely to experience the first relapse, compared with those receiving combination satralizumab-IST therapy, but this difference did not reach statistical significance (see Table).
Table: Hazard ratios for time to first relapse [1]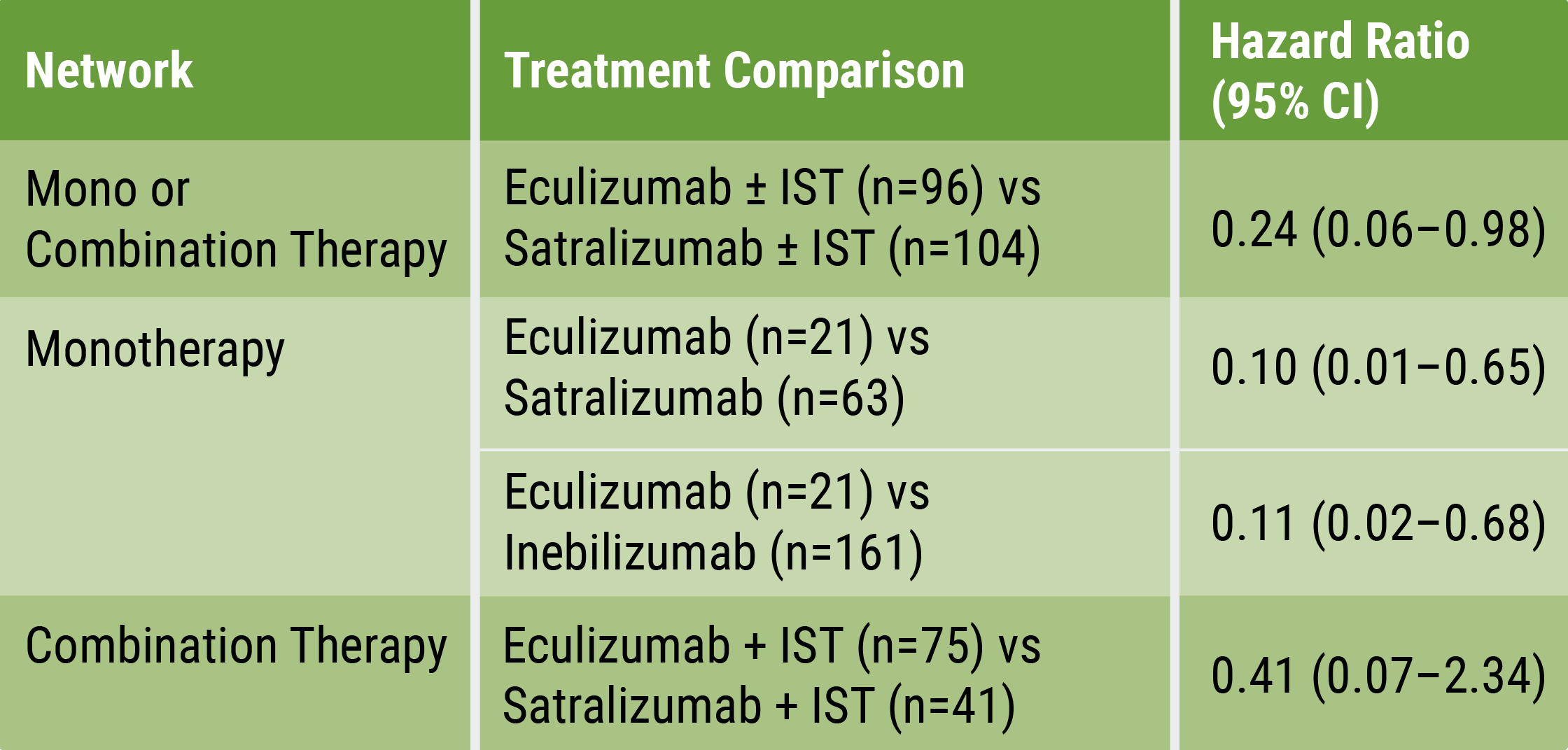 IST, immunosuppressant therapy
IST, immunosuppressant therapy
Prof. Wingerchuck concluded: “Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to be more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings suggest that C5 complement inhibition with treatments such as eculizumab prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD.” However, multiple factors need to be considered in the differential treatment decision process [3].
- Wingerchuck DM, et al. Indirect comparison analysis of FDA-approved treatment options for adults with aquaporin-4 immunoglobulin G-positive neuromyelitis optica spectrum disorder. OP118, ECTRIMS 2021 Virtual Congress, 13–15 October.
- Wingerchuck DM, et al. Neurol Ther 2021;Nov 13. DOI:10.1007/s40120-021-00295-8
- Hartung HP. Ann Neurol. 2021 Jun;89(6):1084–1087.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Long-term efficacy of satralizumab for NMOSD Next Article
Ocrelizumab shows long-term benefits in primary progressive MS »
« Long-term efficacy of satralizumab for NMOSD Next Article
Ocrelizumab shows long-term benefits in primary progressive MS »
Table of Contents: ECTRIMS 2021
Featured articles
Preliminary data shows positive results of ATA188 for progressive MS
COVID-19
MS patients at risk of hampered immune response after vaccination
Immunotherapy in MS does not influence COVID-19 severity and mortality
Anti-CD20 antibodies associated with worse COVID-19 outcomes
ECTRIMS-EAN consensus on vaccination in MS patients
Experimental Treatments
The role of astrocyte phenotypes in acute MS lesions
Promising results of intrathecal MSC-NTF cells in progressive MS
Preliminary data shows positive results of ATA188 for progressive MS
Evobrutinib reduces relapses and MRI lesion activity
Primary endpoint of opicinumab for relapsing MS not met in AFFINITY trial
Elezanumab did not outperform placebo in progressive and relapsing MS
Ibudilast reduced retinal atrophy in primary progressive MS
Treatment Trials and Strategies
ECTRIMS/EAN Clinical Guidelines on MS treatment: an update
Rituximab most effective initial MS therapy in Swedish real-world study
Ublituximab meets primary endpoint for relapsing MS
Dynamic scoring system aids decision to switch MS therapies early
Long-term suppression of MRI disease activity with ocrelizumab
Stopping DMT: when or if at all?
Biomarkers
Early predictors of disability progression in paediatric-onset MS
High-sensitive biomarker detection in MS via novel ELISA assay
Cortical lesions predict cognitive impairment 20 years after MS diagnosis
Applicability of sNfL measurement in clinical practice
MRI more sensitive for disease activity than relapses in SPMS
Imaging
Changes in GABA-receptor binding among cognitively impaired MS patients
T2 lesions independently predict early conversion to SPMS
Natural killer-like CD8+ T cells as a reservoir of clonal cells related to MS activity
Neuromyelitis Optica Spectrum Disorder (NMOSD)
Eculizumab, satralizumab, or inebilizumab for NMOSD?
Long-term efficacy of satralizumab for NMOSD
Long-term efficacy data: inebilizumab for NMOSD
Progressive MS
Charcot Award 2021: Progressive MS, a personal perspective
Top score poster: Meta-analysis on the effect of DMTs
Cortical lesions predict disease progression and disability accumulation
Ocrelizumab shows long-term benefits in primary progressive MS
Other
WNT9B-gene variant associated with doubled relapse risk in MS
Melatonin associated with improved sleep quality in MS patients
“Expanded Disability Status Scale 0 is not normal”
Personality trait alterations in MS patients
Related Articles
November 18, 2024
AI versus clinicians: who diagnoses MS faster and better?
December 9, 2021
Long-term suppression of MRI disease activity with ocrelizumab
December 20, 2022
COVID-19 and MS: lessons learned thus far
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

