https://doi.org/10.55788/556fd2c8
Migraine patients usually have an early response, within 12 weeks of treatment, to CGRP inhibitors. A real-life cohort study, presented by Dr Cinzia Aurilia (University of Florence, Italy), was set up to establish the late response rate (beyond 12 weeks of treatment) in all consecutive patients who were treated with a CGRP inhibitor for ≥12 months over a 3-year period [1].
Included were 912 patients from 16 specialised centres with either chronic migraine (CM; n=690) or high-frequency episodic migraine (HFEM; n=222). Patients were treated with erenumab (n=789), fremanezumab (n=65), or galcanezumab (n=58). The primary endpoint was the proportion of late responders (≥50% response >12 weeks) and the secondary endpoint was the estimated median week of response in these patients.
Overall, 352 patients (38.6%) were non-responders at week 12. From the erenumab, fremanezumab, and galcanezumab receivers, 43.6% (344), 21.5% (14), and 24.1% (14) were non-responders, respectively. Of these non-responders, just over a third (36.4%; n=128) had a response at a later stage: erenumab 33.1% (n=114), fremanezumab 35.7% (n=5), galcanezumab 64.2% (n=9). Rates of responders, non-responders, and late responders for all 3 therapies are shown in the Figure. In the group of late responders, a ≥50% response was seen after a median of 20 weeks (IQR 4–24) of treatment: 20, 16, and 20 weeks in the erenumab, fremanezumab, and galcanezumab group, respectively.
Figure: Rates of responders, non-responders, and late responders for erenumab, fremanezumab, and galcanezumab [1]
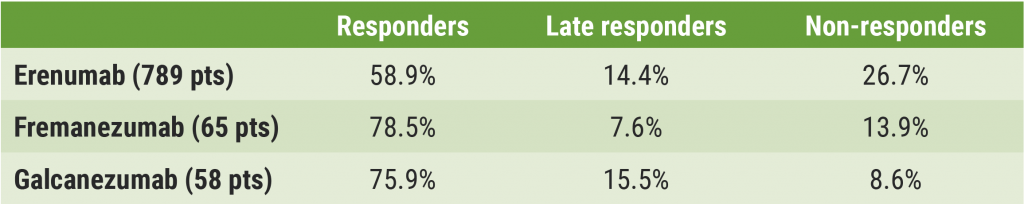
Pts, patients.
Based on these results, the authors propose to continue evaluating the efficacy of a CGRP inhibitor for up to 6 months and to align reimbursement policies accordingly.
- Aurilia C, et al. Late response to anti-CGRP (calcitonin gene-related peptide) monoclonal antibodies: implication for clinical practice. OPR-050, EAN 2022, 25–28 April, Vienna, Austria.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Getting evidence into practice Next Article
Erenumab more than doubles plasma CGRP levels »
« Getting evidence into practice Next Article
Erenumab more than doubles plasma CGRP levels »
Table of Contents: EAN 2022
Featured articles
Letter from the Editor
Overarching Theme
Migraine
Targeting cortical activation by transcranial magnetic stimulation
Erenumab more than doubles plasma CGRP levels
Over a third of patients responds late to CGRP antibodies
Multiple Sclerosis
When to start, switch, and stop MS therapy: Real-world evidence counts
Updated EAN-ECTRIMS guideline on pharmacological MS treatment
Gut microbiota composition associated with disability worsening
Teriflunomide in children with MS: final results of TERIKIDS
Estimating brain age in MS: machine learning versus deep learning
Ofatumumab improves cognitive processing speed
Parkinson’s Disease
Intestinal alterations in patients with Parkinson’s disease
Gene variants impact survival in monogenic Parkinson’s disease
Cerebrovascular Disease and Stroke
Most acute stroke patients have undiagnosed risk factors
Absence of Susceptibility Vessel Sign points to malignancy in stroke patients
Acute stroke management: from time window to tissue window?
Epilepsy
Seizure forecasting with non- and minimally-invasive devices
Real-world efficacy of cenobamate in focal-onset seizures
Possible new biomarker for early neuronal death in mesial temporal lobe epilepsy
COVID-19
COVID-19 elevates risk of neurodegenerative disorders
More headaches in adolescents during COVID-19 pandemic
AstraZeneca vaccination and risk of cerebral venous sinus thrombosis
Large impact of COVID-19 on dementia diagnosis and care
Miscellaneous
Tau autoimmunity associated with systemic disease
Long-term effects of avalglucosidase alfa in late-onset Pompe disease
European survey of patient satisfaction in the treatment of cancer-related neuropathic pain
Related Articles
November 11, 2021
CTA identifies stroke patients most likely to benefit from thrombectomy
January 5, 2023
Large gaps worldwide in prophylactic treatment of migraine
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

