Advanced, recurrent endometrial cancer has a dismal prognosis. Carboplatin and paclitaxel are considered to be the first-line systemic treatment of choice. However, no standard treatment has been defined yet for second- or third-line systemic treatment. In the phase 2 PALEO trial, 77 patients with primary stage 4 or relapsed ER-positive endometrial cancer who had 1 or more previous lines of therapy, were enrolled [1]. Prior surgery, radiation therapy, chemotherapy or ≤1 line of endocrine therapy was permitted. Patients were 1:1 randomised to receive letrozole (2.5 mg) plus placebo or letrozole plus palbociclib (125 mg) until progression.
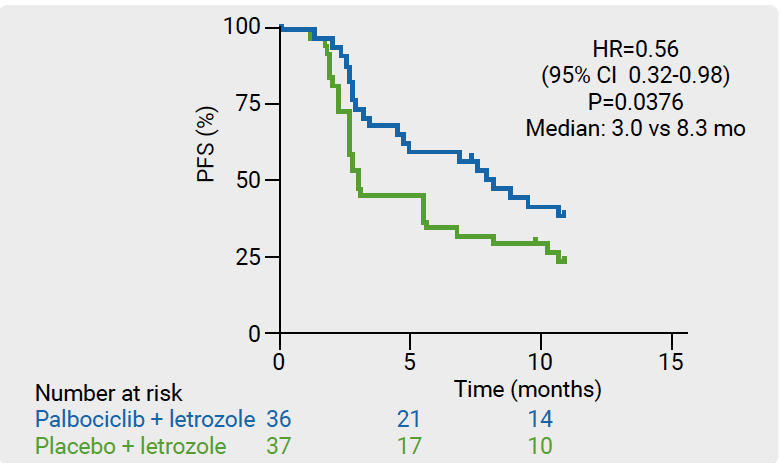
Letrozole plus palbociclib significantly improved PFS compared with letrozole plus placebo (median PFS 8.3 vs 3.0 months; HR 0.56; 95% CI 0.32-0.98; P=0.038; see Figure). The secondary endpoint, disease control rate at 24 weeks, was also in favour of letrozole plus palbociclib (64% vs 38%). Treatment-emergent grade 3/4 adverse events were significantly more frequent with letrozole plus palbociclib compared with letrozole plus placebo (neutropenia 42% vs 0%; anaemia 8% vs 3%).
Figure: Progression-free survival in ITT population of NSGO-PALEO/ENGOT-EN3 trial [1]
- Mirza MR, et al. A randomised double-blind placebo-controlled phase II trial of palbociclib combined with letrozole (L) in patients (pts) with oestrogen receptor-positive (ER+) advanced/recurrent endometrial cancer (EC): NSGO-PALEO / ENGOT-EN3 trial. ESMO 202 Virtual, abstract LBA28.
Posted on
Previous Article
« Bispecific antibodies targeting PD-1 and CTLA-4: new kids on the block(ade) Next Article
Treatment of recurrent ovarian cancer »
« Bispecific antibodies targeting PD-1 and CTLA-4: new kids on the block(ade) Next Article
Treatment of recurrent ovarian cancer »
Table of Contents: ESMO 2020
Featured articles
COVID-19 and Cancer
Breast Cancer
Gastrointestinal Cancers
Lung Cancer
Melanoma
Genitourinary Cancers
Bladder cancer risk and early detection
Gynaecological Cancers
Basic Science
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

