Pembrolizumab was the first anti-PD-1 antibody monotherapy to improve survival in patients with metastatic NSCLC in first line [1]. At 5-year follow-up, 99/150 (66%) patients treated with chemotherapy had crossed over to pembrolizumab, whereas 80/154 (52%) of patients in the pembrolizumab group received subsequent anti-cancer therapy including 12 who received a second course of pembrolizumab [2]. Reported 5-year overall survival (OS) rate in the pembrolizumab arm was 31.9% versus 16.3% in the chemotherapy arm, despite 66% cross-over. Progression-free survival (PFS) rate at 5 years was 22.8% and 4.1%, respectively. Objective response rate (ORR) in the patients treated with pembrolizumab was 46.1% (4.5% complete, 41.6% partial response) versus 31.1% (all partial response) in patients treated with chemotherapy. Median duration of response was 29.1 months and 6.3 months, respectively. In the pembrolizumab arm, 39 patients completed 2 years treatment with pembrolizumab. In these patients, 3-years OS rate from completion was 81% and ORR was 82% (10% complete, 72% partial response); 18/39 (46%) were alive without progression of disease at data cut-off.
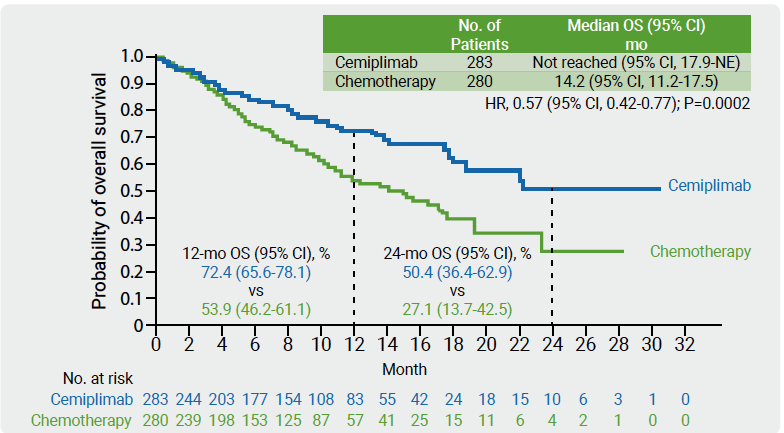
Cemiplimab is a high-affinity anti-PD-1 antibody demonstrating anti-tumour activity in phase 1 and phase 2 trials with a safety profile similar to that described for other PD-1 inhibitors in several advanced solid tumours including NSCLC [3]. In the phase 3 EMPOWER-Lung 1 trial, 710 treatment-naïve patients were randomised 1:1 to receive cemiplimab (350 mg every 3 weeks) or investigator’s choice of chemotherapy. At data cut-off, 74% patients who progressed on chemotherapy had crossed-over from chemotherapy to cemiplimab. A prespecified interim analysis was performed after 50% of OS events [4]. In the ITT population, median OS was 22.1 months with cemiplimab (n=356) versus 14.3 months with chemotherapy (n=354; HR 0.68; P=0.002). 2-years OS rate was 48.6 and 29.7 months, respectively. In the PD-L1 ≥50% ITT population, median OS was not reached with cemiplimab (n=283) versus 14.2 months with chemotherapy (n=280; HR 0.57; P=0.0002; see Figure). Grade ≥3 adverse events were lower in patients treated with cemiplimab compared with chemotherapy (37.2% vs 48.5%).
Figure: Overall survival in the PD-L1 ≥50% ITT population of EMPOWER-Lung 1 [4]
- Reck M, et al. N Engl J Med 2016;375:1823-1833.
- Brahmer J, et al. KEYNOTE-024 5-year OS update: First-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥50%. ESMO 2020 Virtual Meeting, abstract LBA51.
- Papadopoulos KP, et al. Clin Cancer Res 2020;26:1025-1033.
- Sezer A, et al. EMPOWER-Lung 1: Phase III first-line (1L) cemiplimab monotherapy vs platinum-doublet chemotherapy (chemo) in advanced non-small cell lung cancer (NSCLC) with programmed cell death-ligand 1 (PD-L1) ≥50%. ESMO 2020 Virtual Meeting, abstract LBA52.
Posted on
Previous Article
« PD-L1 density, a new predictive biomarker in NSCLC Next Article
Lorlatinib outperforms crizotinib in ALK-positive advanced NSCLC »
« PD-L1 density, a new predictive biomarker in NSCLC Next Article
Lorlatinib outperforms crizotinib in ALK-positive advanced NSCLC »
Table of Contents: ESMO 2020
Featured articles
COVID-19 and Cancer
Breast Cancer
Gastrointestinal Cancers
Lung Cancer
Melanoma
Genitourinary Cancers
Bladder cancer risk and early detection
Gynaecological Cancers
Basic Science
Related Articles
November 25, 2020
First-line immune-combination therapies in mUC
November 25, 2020
Palbociclib effective in ER-positive endometrial cancer
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

