The presented phase 2b trial included 204 adult patients with moderate-to-severe AD. For 12 weeks, 3 randomised groups of study subjects were treated with either 45 mg, 150 mg, or 300 mg of tralokinumab every second week, with a fourth group receiving a placebo. All patients were on a background treatment with topical corticosteroids (WHO class 3). These steroids were applied ≥1 time daily during 2 weeks before receiving tralokinumab, and, if necessary, throughout the rest of the trial. Efficacy was assessed by the percentage of subjects reaching an Investigator’s Global Assessment (IGA) score of 0 or 1, corresponding to clear or almost clear skin with a ≥2-point decrease from baseline and changes in Eczema Area Severity Index (EASI) (co-primary endpoints).
Influence on microbiome and biomarkers
To gain further insight, data was also collected on the intensity of colonisation with Staphylococcus aureus, which can trigger disease flares. Several potential AD-associated biomarkers, such as immunoglobulin E (IgE), periostin, chemokine ligand 17 (CCL17), and dipeptidyl peptidase-4 (DPP-4) were also measured. The application of the highest tested dosage of tralokinumab (300 mg) resulted in a significant reduction of -4.9 in EASI at week 12 (P=0.01). Compared with placebo, nearly 18% more patients reached the IGA response (26.7% vs 11.8%).
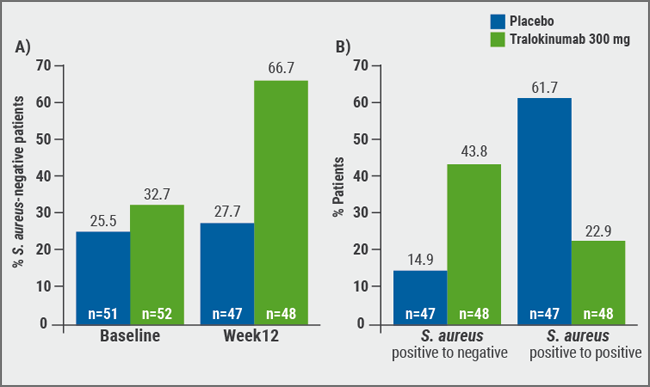
Abundance of Staphylococcus colonisation and serum levels of CCL17 both correlated with disease severity (EASI) at baseline. A negative status for S. aureus in lesional skin at week 12 was seen in 66.7% of tralokinumab patients, compared with 27.7 % of patients who received placebo (see Figure). Patients treated with tralokinumab showed decreased levels of periostin (-31.3% vs +1.9%), CCL17 (-40.0% vs +37.4%), and IgE (-22.3% vs +1.6%) compared with placebo, while DDP-4 was slightly increased (+7.3% vs +3.9%). Upper respiratory infections were the most common treatment-emergent adverse events in both groups. They occurred in 3.9% of patients in the pooled tralokinumab dose groups compared with 3.9% of placebo patients. Headaches appeared in both 2% of pooled tralokinumab and placebo groups.
In conclusion, tralokinumab not only induced clinical improvement but also decreased S. aureus and several serum biomarkers. Greatest improvements with tralokinumab therapy were achieved in patients with high DPP-4, low periostin, and a positive status for S. aureus at baseline.
Figure: Proportion of patients with A) S. aureus-negative lesional skin at baseline and at week 12, and B) shift from S. aureus-positive at baseline to S. aureus-negative at week 12 and S. aureus-positive both at baseline and week 12 in lesional skin [1]
1. Guttman-Yassky E et al. ePoster No. 8690, AAD Annual Meeting, 1-5 March 2019, Washington DC, USA.
Posted on
Previous Article
« New standardised cantharidin product against molluscum contagiosum efficacious in two phase 3 trials Next Article
Thicker AK lesions benefit from laser pretreatment with high channel density »
« New standardised cantharidin product against molluscum contagiosum efficacious in two phase 3 trials Next Article
Thicker AK lesions benefit from laser pretreatment with high channel density »
Table of Contents: AAD 2019
Featured articles
Letter from the Editor
Interview with AAD president Prof. George J. Hruza
Late-Breakers
Secukinumab maintains improvements in psoriasis through 5 years of treatment
Bermekimab – a future treatment for atopic dermatitis?
JAK1/2 inhibitor effective in alopecia areata
Novel anti-IgE drug enables durable urticaria control
Dual IL-17A and IL-17F blocker leads to unprecedented response rates in psoriasis
Thicker AK lesions benefit from laser pretreatment with high channel density
New standardised cantharidin product against molluscum contagiosum efficacious in two phase 3 trials
Bruton’s tyrosine kinase inhibitor highly effective in pemphigus vulgaris
Serlopitant reduces pruritus associated with psoriasis
Atopic Dermatitis: Many New Therapies in the Pipeline
New and emerging atopic dermatitis therapies
Food triggers eczema – an imperturbable belief of patients
Psoriasis and Biologics: The Beat Goes On
Psoriasis and Biologics: The Beat Goes On
JAK Inhibitors: A New Frontier in Dermatology
JAK inhibitors: a new therapeutic tool for dermatologists
JAK inhibitors: a pathogenesis-directed therapy for alopecia areata
Can JAK inhibitors close the current therapeutic gap in AD?
Hair Loss: No Reason for Therapeutic Nihilism
Hair Loss: No Reason for Therapeutic Nihilism
Vitiligo: The Beginning of a New Era
Vitiligo in children
Surgical treatment for selected vitiligo cases
JAK-inhibitors: an emerging treatment option for vitiligo
What's New and Hot in Acne
Should we use more hormonal therapy?
Pearls of the Posters
Pemphigus patients prone to osteoporosis
Intralesional 5-fluorouracil induced high clearance rates in cutaneous squamous cell carcinoma
Related Articles

August 28, 2020
Adjuvant pembrolizumab: durable RFS for stage III melanoma
November 19, 2021
IFN-γ signature predicts response to immunotherapy

© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

