ALTA-1L was an open-label, phase 3 trial that compared the safety and efficacy of brigatinib vs crizotinib in patients with ALK inhibitor-naïve ALK+ advanced NSCLC. The initial interim analysis showed the trial met the primary endpoint: PFS was significantly longer in patients who received brigatinib vs crizotinib, marking the former as a promising first-line agent for ALK-positive NSCLC. Dr Ross Camidge (University of Colorado Cancer Center, USA) presented early results [6] and the study was simultaneously published in the New England Journal of Medicine [7].
Brigatinib is a next-generation ALK inhibitor that targets a broad range of ALK mutations and ROS1 rearrangements [7]. The ALTA-1L study enrolled 275 patients with advanced ALK+ NSCLC, Eastern Cooperative Oncology Group (ECOG) performance status 0-2, and less than one prior systemic anticancer regimen. Patients with asymptomatic, untreated central nervous system (CNS) metastasis were eligible for the clinical trial.
Exclusion criteria included prior treatment with an ALK inhibitor, or radiation therapy within 14 days of the first dose of brigatinib. Patients were stratified according to baseline brain metastases and completion of at least one full cycle of prior chemotherapy and were randomly assigned to receive either brigatinib (n=137) or crizotinib (n=138). Brigatinib was initiated at a lead-in dose 90 mg for 1 week, followed by 180 mg once daily (QD). Crizotinib was given at a standard dose 250 mg twice a day. The primary endpoint was PFS determined by a blinded independent review committee. Overall, 27% of the cohort had received prior chemotherapy and 29% had baseline brain metastases. At the time of the interim analysis, median follow-up was 11.0 months among patients who received brigatinib and 9.3 months in the crizotinib-treated patients. Estimated 12-month PFS rates were higher in the former than in the latter—67% vs 43% (HR 0.49; P<0.001; see Figure) [7].
Figure: Primary endpoint of progression-free survival assessed by blinded investigational review committee [6]
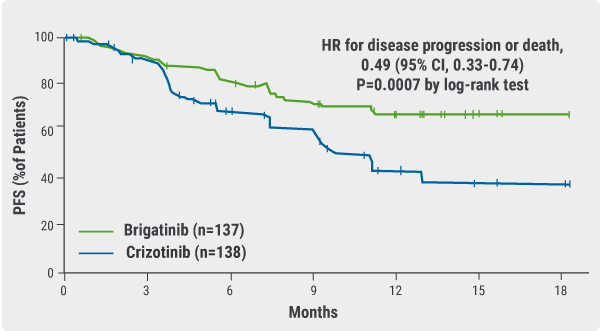
The confirmed objective response rate (ORR) was 71% vs 60% for brigatinib and crizotinib, respectively and the confirmed rates of intracranial response in patients with measurable lesions were 78% vs 29%. In patients without prior chemotherapy, brigatinib reduced the risk of disease progression or death by 45% compared with crizotinib (HR 0.55). In patients who had prior chemotherapy, it reduced the risk by 65% vs crizotinib (HR 0.35; P=0.0207). In patients with brain metastases at baseline, the risk of progression or death with brigatinib was 73% lower than that with crizotinib (HR 0.27; P<0.0001). There were no new safety concerns reported.
- Camidge DR, et al. Abtract PL02.03. IASLC 19th WCLC. 23-26 September 2018, Toronto, Canada.
- Camidge DR, et al. N Engl J Med, September 25, 2018.
Posted on
Previous Article
« Accumulation of concomitant mutations involved in drug resistance in sequential ALK TKI treatments of ALK-positive NSCLC Next Article
Angiogenic and stromal biomarkers in malignant mesothelioma »
« Accumulation of concomitant mutations involved in drug resistance in sequential ALK TKI treatments of ALK-positive NSCLC Next Article
Angiogenic and stromal biomarkers in malignant mesothelioma »
Table of Contents: WCLC 2018
Featured articles
Interview with the IASCL President, Dr. Giorgio Scagliotti
Presidential Symposium – Top 5 abstracts
Durvalumab after chemoradiotherapy extends OS in stage 3, unresectable non-small-cell lung cancer
Potential for brigatinib as a first-line treatment option for ALK+ non-small-cell lung cancer
Benefits of chest CT screening
New standard of care in extensive-stage small-cell lung cancer
No progression-free survival benefit with nintedanib plus pemetrexed/cisplatin for malignant pleural mesothelioma of epithelial subtype
New Aspects of Immunotherapy
Next generation immunotherapy in non-small-cell lung cancer
Combination therapies: Where are we in 2018?
Choice of taxane and addition of pembrolizumab for metastatic squamous non-small-cell lung cancer
New Aspects of Targeted Therapy
PD-L1 expression in untreated EGFR-mutant non-small-cell lung cancer and response to osimertinib
Mesothelioma
Unmet needs in surgical management of malignant pleural mesothelioma
Advanced Non-small Cell Lung Cancer
Novel Therapies in ROS1 and EGFR
Advances in Small-cell and Neuroendocrine Tumours
Related Articles
November 21, 2018
Benefits of chest CT screening
November 21, 2018
New standard of care in extensive-stage small-cell lung cancer
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

