ALK rearrangement detection using fluorescence in situ hybridisation (FISH) or dichotomous immunohistochemical staining (IHC) is a standard test to identify patients with NSCLC eligible for treatment with ALK inhibitors [7].
A European, prospective, multicentre study compared response to treatment with crizotinib in ALK FISH+ and ALK FISH- in patients with ALK IHC+. Central collection of stage 4 ALK IHC+ NSCLC cases treated with crizotinib took place from April 2014 to November 2017. Slides were centrally validated for ALK IHC and ALK FISH. Monthly recording showed that of 3,523 registered ALK IHC tests, 94 (2.6%) were ALK IHC+; less than 0.01% were ALK IHC+ FISH- cases. Local ALK FISH analysis identified 46 concordant (ALK IHC+/FISH+) cases and 18 that were discordant (ALK IHC+/FISH-).
Central validation in Antwerp, Belgium, found 37 concordant and 6 discordant cases, 5 of which had follow-up. Limited tissue in the biopsy samples hampered validation. Time to treatment was the same for concordant and discordant cases (HR 0.78; P=0.64). The same was true for local or validated ALK testing (HR 2.2; P=0.16). But, after central validation, overall survival was significantly longer for patients with concordant cases than discordant ones (HR 4.5; P=0.010; see Figure). Local testing found otherwise (HR 1.7; P=0.44)
Figure: Overall survival based on central testing
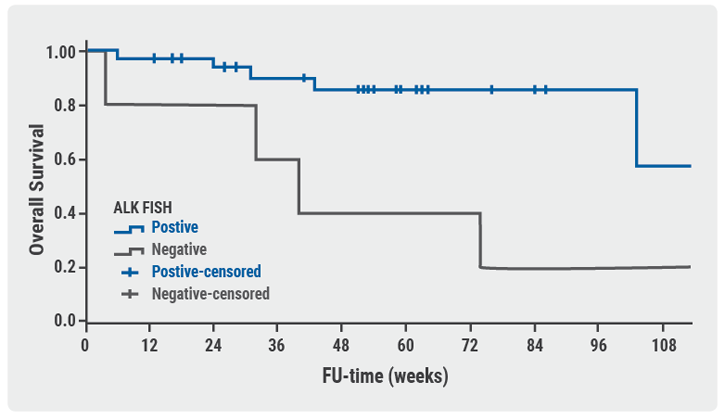
- van der Wekken AJ, et al. Clin Cancer Res 2017;23:4251-4258.
Posted on
Previous Article
« Interview with the IASCL President, Dr. Giorgio Scagliotti Next Article
Benefits of chest CT screening »
« Interview with the IASCL President, Dr. Giorgio Scagliotti Next Article
Benefits of chest CT screening »
Table of Contents: WCLC 2018
Featured articles
Interview with the IASCL President, Dr. Giorgio Scagliotti
Presidential Symposium – Top 5 abstracts
Durvalumab after chemoradiotherapy extends OS in stage 3, unresectable non-small-cell lung cancer
Potential for brigatinib as a first-line treatment option for ALK+ non-small-cell lung cancer
Benefits of chest CT screening
New standard of care in extensive-stage small-cell lung cancer
No progression-free survival benefit with nintedanib plus pemetrexed/cisplatin for malignant pleural mesothelioma of epithelial subtype
New Aspects of Immunotherapy
Next generation immunotherapy in non-small-cell lung cancer
Combination therapies: Where are we in 2018?
Choice of taxane and addition of pembrolizumab for metastatic squamous non-small-cell lung cancer
New Aspects of Targeted Therapy
PD-L1 expression in untreated EGFR-mutant non-small-cell lung cancer and response to osimertinib
Mesothelioma
Unmet needs in surgical management of malignant pleural mesothelioma
Advanced Non-small Cell Lung Cancer
Novel Therapies in ROS1 and EGFR
Advances in Small-cell and Neuroendocrine Tumours
Related Articles
November 21, 2018
Benefits of chest CT screening
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

