The spectrum of acquired resistance to osimertinib is not yet fully characterised. Piotrowska et al. investigated RET-specific TKI BLU-667 and osimertinib in a cohort of acquired resistance biopsies and one patient with RET-mediated acquired resistance [1]. Osimertinib is a selective, CNS-penetrant, third generation EGFR TKI approved by the FDA and EMA for front-line use [2].
BLU-667 is an investigational selective RET inhibitor with clinical activity in NSCLCs and other cancers with RET alterations [3]. The patient in this single-centre study was treated with osimertinib/BLU-667 using an IRB- and FDA-approved compassionate use protocol. Forty-one EGFR-mutant patients with acquired resistance to osimertinib were histologically assessed and screened by tissue (n=22) or plasma next-generation sequencing (n=9), or both (n=10). Fusions in plasma and/or tissue were found in 4 patients treated with osimertinib.
Tissue from one patient had co-occurrent CCDC-RET and TPM3-NTRK1 fusions in circulating tumour DNA. Tissue from the other patients showed fusions in CCDC-RET, PCB2-BRAF, and AGK-BRAF. CCDC6-RET was expressed in EGRFm NSCLC cell lines PC9 (Ex19del) and MGH 134 (L858R/T790M). These maintained MAPK signalling and conferred resistance to osimertinib and afatinib. Inhibition of RET by BLU-667 or cabozantinib resensitised cells expressing CCDC6-RET to EGFR inhibition.
A 60-year-old woman with Ex19del progressed on afatinib (T790M+), then osimertinib. Tissue biopsy at osimertinib acquired resistance found acquired CCDC6-RET (T790-wt). She started osimertinib at 80 mg/BLU-667 200 mg daily for 2 weeks, then BLU-667 at 300 mg daily. Dyspnoea improved within days of starting treatment. Scans after 8 weeks showed a marked response, with RECIST tumour shrinkage of 78% (see Figure).
Figure: Scans after 8 weeks of treatment with osimertinib/BLU-667 showed tumour shrinkage of 78%
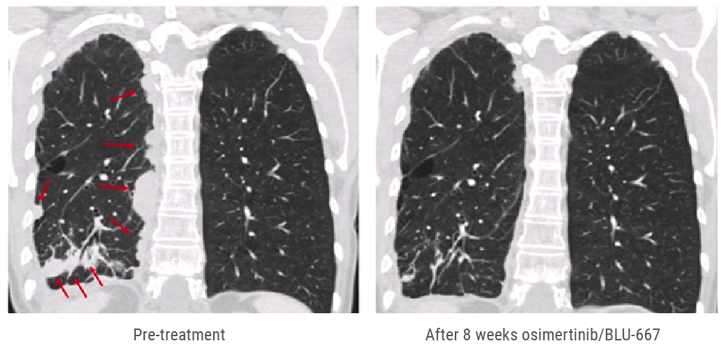
Study outcomes indicate that RET and other oncogene fusions are a recurrent finding in EGFRm NSCLC with acquired resistance to osimertinib and other EGFR TKIs. In vitro models suggest that CCDC6-RET mediates EGFR resistance and can be inhibited by combined EGFR + RET inhibition. This is the first report of a clinical response to combined EGFR + RET inhibition in EGFRm NSCLC and acquired CCDC-RET fusion after osimertinib. At the time of presentation, osimertinib/BLU-667 appeared to be well tolerated, with only grade 1 AEs. Treatment is ongoing.
- Piotrowska Z et al. Cancer Discov 2018 Sep 2 [Epub ahead of print]
- Soria JC, Ohe Y, Vansteenkiste J, et al. N Engl J Med 2018;378:113-125.
- Subbiah V, Gainor JF, Rahal R, et al. Cancer Discov 2018;8:836-849.
Posted on
Previous Article
« Patient-reported outcomes of first-line nivolumab + ipilimumab in high tumour mutational burden advanced non-small-cell lung cancer Next Article
Letter from The Editor »
« Patient-reported outcomes of first-line nivolumab + ipilimumab in high tumour mutational burden advanced non-small-cell lung cancer Next Article
Letter from The Editor »
Table of Contents: WCLC 2018
Featured articles
Interview with the IASCL President, Dr. Giorgio Scagliotti
Presidential Symposium – Top 5 abstracts
Durvalumab after chemoradiotherapy extends OS in stage 3, unresectable non-small-cell lung cancer
Potential for brigatinib as a first-line treatment option for ALK+ non-small-cell lung cancer
Benefits of chest CT screening
New standard of care in extensive-stage small-cell lung cancer
No progression-free survival benefit with nintedanib plus pemetrexed/cisplatin for malignant pleural mesothelioma of epithelial subtype
New Aspects of Immunotherapy
Next generation immunotherapy in non-small-cell lung cancer
Combination therapies: Where are we in 2018?
Choice of taxane and addition of pembrolizumab for metastatic squamous non-small-cell lung cancer
New Aspects of Targeted Therapy
PD-L1 expression in untreated EGFR-mutant non-small-cell lung cancer and response to osimertinib
Mesothelioma
Unmet needs in surgical management of malignant pleural mesothelioma
Advanced Non-small Cell Lung Cancer
Novel Therapies in ROS1 and EGFR
Advances in Small-cell and Neuroendocrine Tumours
Related Articles

November 21, 2018
Next generation immunotherapy in non-small-cell lung cancer
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

