ALK TKIs have shown promising clinical outcomes for ALK-positive lung cancer patients. Response to various TKIs is influenced by distinct resistant-mechanisms for different ALK fusion variants.
An investigation of genomic alterations in homogenous resistant-mechanisms reported their diversity and complexity in response to next-generation ALK TKIs. Cohort 1 included 475 ALK-positive lung cancer patients out of a total 11,842 who underwent next-generation sequencing to assess the distribution of ALK fusion variants. Cohort 2 included 52 NSCLC patients with a similar distribution of ALK fusion variants as those in cohort 1. Mutation profiles of 416 cancer-relevant genes in their post-ALK TKI tumour samples were analysed. The crizotinib-only treatment group included 35 patients; 17 other patients (the multi-TKI group) received multiple lines of ALK TKIs, including lorlatinib, alectinib, ceritinib, and brigatinib.
The two most common ALK fusion variants in in both cohorts were EML4-ALK v3 and v1. In the crizotinib group, 17 (49%) patients had 18 different ALK activating mutations; the number in the multi-TKI group was 10 (59%). Both groups had different mutation patterns. G1202R, found in 35% of patients, was the most frequent ALK activating mutation. L1196M (14%) and G1269A (11%) were more common in the crizotinib cohort. Compared with the latter, there was significant enrichment of concomitant ALK activating mutations in the multi-TKI group (P=0.31).
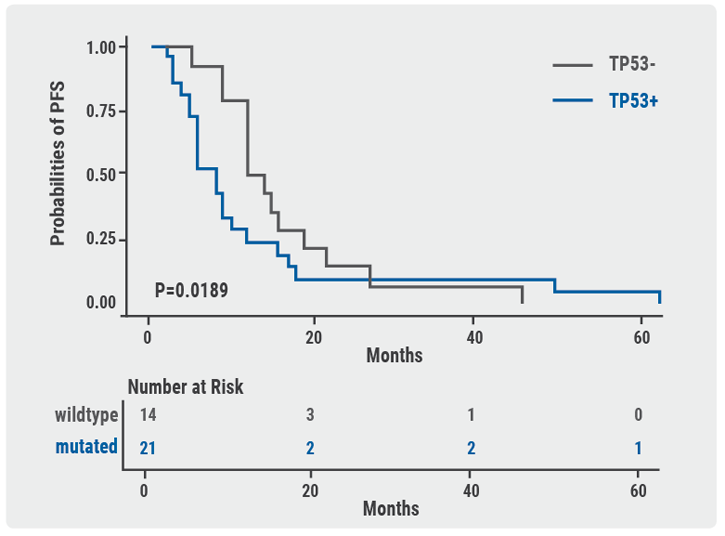
A trend showed an increased number of multi-TKI patients with by-pass/downstream pathway activation of ALK vs the crizotinib cohort (P=0.056). This led to higher recurrence of dual activation of ALK and ALK by-pass/downstream pathways in the multi-TKI (29%) group vs crizotinib-treated patients (6%; P=0.031). Regardless of fusion variant types, crizotinib-treated patients with concomitant TP53 mutation had significantly shorter PFS compared with TP53 wildtype patients. The median PFS was 8 vs 13 months (HR 1.494; P=0.019). These findings suggest a higher frequency of concomitant mutations after multiple TKI treatments. They also indicate that concomitant TP53 mutation might be a biomarker for worse clinical outcomes in patients treated with crizotinib (see Figure). Concomitant ALK activation mutations are more common after multi-TKI treatments. In patients treated with crizotinib alone, TP53 mutation might be a prognostic biomarker of worse clinical outcomes
Figure: TP53 might be a negative prognostic factor in ALK+ NSCLC patients treated with crizotinib alone.
Posted on
Previous Article
« Combination therapies: Where are we in 2018? Next Article
Potential for brigatinib as a first-line treatment option for ALK+ non-small-cell lung cancer »
« Combination therapies: Where are we in 2018? Next Article
Potential for brigatinib as a first-line treatment option for ALK+ non-small-cell lung cancer »
Table of Contents: WCLC 2018
Featured articles
Interview with the IASCL President, Dr. Giorgio Scagliotti
Presidential Symposium – Top 5 abstracts
Durvalumab after chemoradiotherapy extends OS in stage 3, unresectable non-small-cell lung cancer
Potential for brigatinib as a first-line treatment option for ALK+ non-small-cell lung cancer
Benefits of chest CT screening
New standard of care in extensive-stage small-cell lung cancer
No progression-free survival benefit with nintedanib plus pemetrexed/cisplatin for malignant pleural mesothelioma of epithelial subtype
New Aspects of Immunotherapy
Next generation immunotherapy in non-small-cell lung cancer
Combination therapies: Where are we in 2018?
Choice of taxane and addition of pembrolizumab for metastatic squamous non-small-cell lung cancer
New Aspects of Targeted Therapy
PD-L1 expression in untreated EGFR-mutant non-small-cell lung cancer and response to osimertinib
Mesothelioma
Unmet needs in surgical management of malignant pleural mesothelioma
Advanced Non-small Cell Lung Cancer
Novel Therapies in ROS1 and EGFR
Advances in Small-cell and Neuroendocrine Tumours
Related Articles

November 21, 2018
Interview with the IASCL President, Dr. Giorgio Scagliotti
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

