Prof. Suresh Ramalingam (Emory University School of Medicine, Winship Cancer Institute, USA) presented data on PD-L1 expression in untreated EGFR-mutant (EGFRm) advanced NSCLC. He also discussed response to osimertinib compared with standard EGFR tyrosine kinase inhibitors (TKIs) in the phase 3 FLAURA trial [1]. “Where does immunotherapy fit in patients with EGFR mutations and other driver events?” asked Prof. Ramalingam. “This is a key, clinically important question.”
Osimertinib is an oral, third-generation, CNS-active EGFR TKI that potently and selectively inhibits both EGFR TKI sensitising and EGFR T790M resistance mutations [1-5]. “The benefit of EGFR TKIs in treatment-naïve, EGFRm, PD-L1 positive patients is of high clinical interest,” Prof. Ramalingam said. A recent study of pembrolizumab in EGFR TKI-naïve patients with EGFRm and PD-L1 positive-advanced NSCLC failed to demonstrate efficacy [6]. “It was halted early,” said Prof. Ramalingam.
However, the FLAURA trial showed that osimertinib significantly improved PFS compared to standard of care EGFR TKIs in patients with untreated Ex19de/L858R-positive (EGFRm) NSCLC [1].
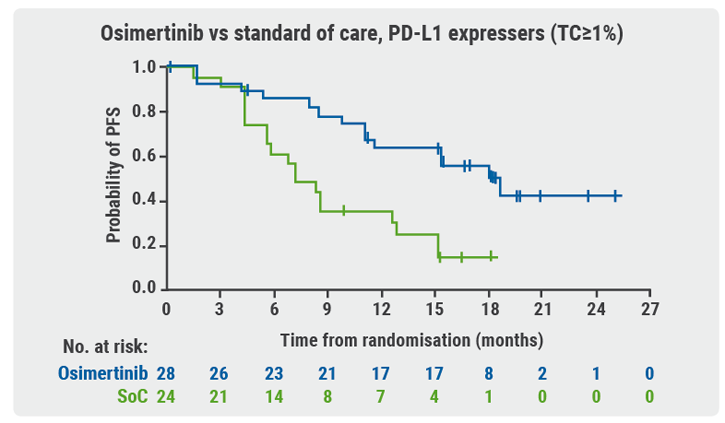
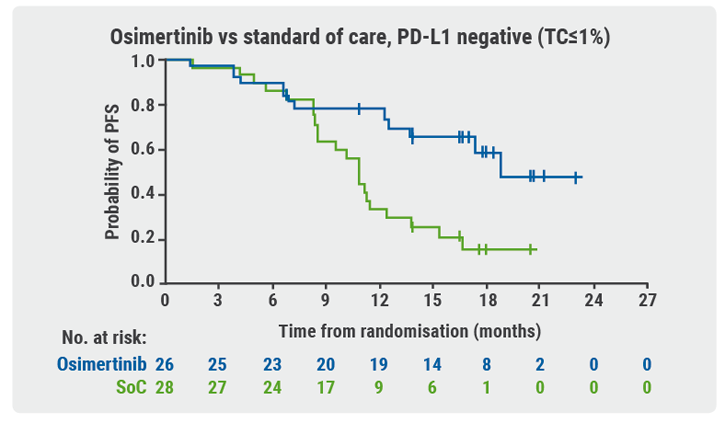
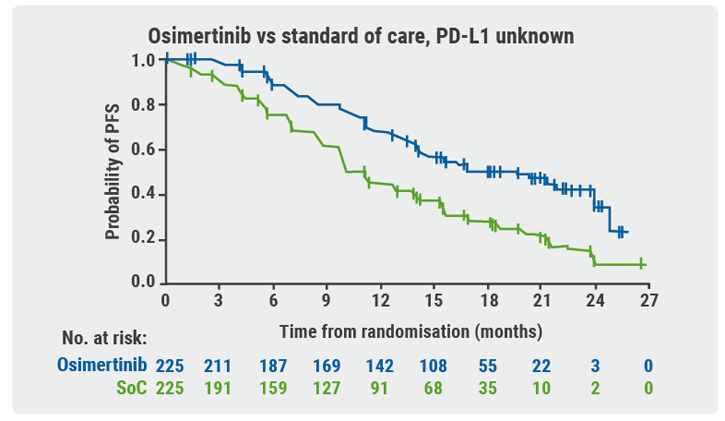
Participants with locally advanced/metastatic NSCLC who were positive for EGFRm and PD-L1 were stratified by mutation type and race (Asian/non-Asian) before being randomised to treatment. Exploratory endpoints included measurement of PD-L1 expression in freshly sectioned archival tumour tissue samples and assessment of clinical outcome by PD-L1 expression. Samples with tumour cell (TC) staining ≥1% were defined as PD-L1 expressers.
PD-L1 expression was lower in EGFRm-positive samples than in EGRFm-negative ones. It was also uncommon at higher thresholds (TC≥25% or TC≥50%) in EGRFm-positive samples. There were no progression-free survival (PFS) assessments in patients randomised to treatment with PD-L1 TC≥25% or TC≥50% tumours; there were too few of them to perform the analysis.
Patients who received osimertinib showed PFS benefit regardless of PD-L1 expression (see Figure). According to Prof. Ramalingam, these outcomes support the following conclusions:
- EGFRm testing is critical prior to treatment initiation in all patients with advanced NSCLC; and
- osimertinib should be used as first-line treatment of patients with EGFRm advanced NSCLC, regardless of PD-L1 status.
- Soria JC, et al. N Engl J Med 2018;378:113-125.
- Cross DA, et al. Cancer Discov 2014;4:1046-1061.
- Mok TS, et al. GN Engl J Med 2017;376:629-640.
- Wu YL, et al. J Clin Oncol 2018:JCO2018777326.
- Reungwetwattana T, et al. J Clin Oncol 2018:JCO2018783118.
- Lisberg A, et al. J Thorac Oncol 2018;13:1138-1145.
Posted on
Previous Article
« Letter from the Editor Next Article
Durvalumab after chemoradiotherapy extends OS in stage 3, unresectable non-small-cell lung cancer »
« Letter from the Editor Next Article
Durvalumab after chemoradiotherapy extends OS in stage 3, unresectable non-small-cell lung cancer »
Table of Contents: WCLC 2018
Featured articles
Interview with the IASCL President, Dr. Giorgio Scagliotti
Presidential Symposium – Top 5 abstracts
Durvalumab after chemoradiotherapy extends OS in stage 3, unresectable non-small-cell lung cancer
Potential for brigatinib as a first-line treatment option for ALK+ non-small-cell lung cancer
Benefits of chest CT screening
New standard of care in extensive-stage small-cell lung cancer
No progression-free survival benefit with nintedanib plus pemetrexed/cisplatin for malignant pleural mesothelioma of epithelial subtype
New Aspects of Immunotherapy
Next generation immunotherapy in non-small-cell lung cancer
Combination therapies: Where are we in 2018?
Choice of taxane and addition of pembrolizumab for metastatic squamous non-small-cell lung cancer
New Aspects of Targeted Therapy
PD-L1 expression in untreated EGFR-mutant non-small-cell lung cancer and response to osimertinib
Mesothelioma
Unmet needs in surgical management of malignant pleural mesothelioma
Advanced Non-small Cell Lung Cancer
Novel Therapies in ROS1 and EGFR
Advances in Small-cell and Neuroendocrine Tumours
Related Articles
November 21, 2018
Small-cell lung cancer circulating tumour cell derived explants
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

