Positive PFS findings from phase 2 of the global randomised LUME-Meso trial did not carry through to phase 3 outcomes as the study failed to meet the primary endpoint of PFS in patients with malignant pleural mesothelioma (MPM) treated with nintedanib plus pemetrexed/cisplatin. IASLC president Prof. Giorgio Scagliotti (University of Turin and San Luigi Hospital, Italy) presented the findings [13].
Nintedanib is a multikinase inhibitor that targets VEGF receptors 1–3, PDGF receptors α/β, FGF receptors 1–3, and Src and Abl kinase signalling [14, 15]. Combined with pemetrexed/ cisplatin, it improved PFS (HR 0.56) with a trend toward prolonged OS (HR 0.77) in phase 2 of the LUME-Meso trial [16]. The effect was particularly evident in patients with epithelioid histology (PFS HR 0.51; OS HR 0.70).
The randomised LUME-Meso study enrolled 458 patients with treatment-naïve, histologically confirmed, unresected epithelioid MPM. Patients received up to 6 cycles of pemetrexed (500 mg2)/cisplatin (75 mg2) on day 1, plus nintedanib (200 mg twice daily) or placebo on days 2-21. Maintenance therapy for patients with no progression after combination treatment was either nintedanib or placebo. The median duration of nintedanib treatment was 5.3 vs 5.1 months for nintedanib and placebo, respectively. After 250 events, no statistically significant differences were observed between the nintedanib and placebo arms in PFS or interim OS. There were no unexpected safety findings, and as per study protocol, the trial was discontinued. MPM is a global epidemic [17].
Since 2003, pemetrexed/cisplatin has been the only approved regimen, with a median OS of approximately 1 year [18]. “This trial reaffirms the need for solid confirmatory studies that are adequately sized to challenge the standard of care in advanced malignant mesothelioma,” said Prof. Scagliotti.
- Scagliotti GV, et al. Abstract PL02.09. IASLC 19th WCLC. 23-26 September, 2018. Toronto, Canada.
- Awasthi N, Schwarz RE. Onco Targets Ther 2015;8:3691-3701.
- Hilberg F, et al. Cancer Res 2008;68:4774-4782.
- Grosso F, et al. J Clin Oncol 2017;35:3591-3600.
- Bibby AC, et al. Eur Respir Rev 2016;25:472-486.
- Vogelzang NJ, et al. J Clin Oncol 2003;21:2636-2644.
Posted on
Previous Article
« Letter from the Editor Next Article
New standard of care in extensive-stage small-cell lung cancer »
« Letter from the Editor Next Article
New standard of care in extensive-stage small-cell lung cancer »
Table of Contents: WCLC 2018
Featured articles
Interview with the IASCL President, Dr. Giorgio Scagliotti
Presidential Symposium – Top 5 abstracts
Durvalumab after chemoradiotherapy extends OS in stage 3, unresectable non-small-cell lung cancer
Potential for brigatinib as a first-line treatment option for ALK+ non-small-cell lung cancer
Benefits of chest CT screening
New standard of care in extensive-stage small-cell lung cancer
No progression-free survival benefit with nintedanib plus pemetrexed/cisplatin for malignant pleural mesothelioma of epithelial subtype
New Aspects of Immunotherapy
Next generation immunotherapy in non-small-cell lung cancer
Combination therapies: Where are we in 2018?
Choice of taxane and addition of pembrolizumab for metastatic squamous non-small-cell lung cancer
New Aspects of Targeted Therapy
PD-L1 expression in untreated EGFR-mutant non-small-cell lung cancer and response to osimertinib
Mesothelioma
Unmet needs in surgical management of malignant pleural mesothelioma
Advanced Non-small Cell Lung Cancer
Novel Therapies in ROS1 and EGFR
Advances in Small-cell and Neuroendocrine Tumours
Related Articles
November 21, 2018
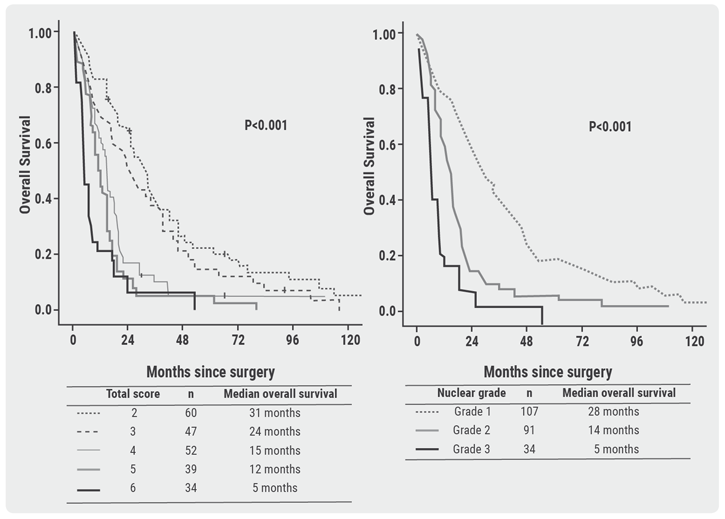
Unmet needs in surgical management of malignant pleural mesothelioma
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

