AA is a poorly treatable autoimmune disease that affects women, men, and children of all ages. This chronic, relapsing, inflammatory disorder is characterised by non-scarring hair loss on the scalp and/or body and has a remarkable negative impact on quality of life. Prof. Taisuke Ito (Hamamatsu University School of Medicine, Japan) explained that hair follicles of healthy subjects have an immune privilege, and that loss of this immune privilege leads to the disease [1]. Certain triggers can lead to activation of NKG2D-expressing CD8+ cytotoxic T lymphocytes, which are necessary for induction of disease. They lead to a type 1 inflammation with upregulation of interleukin (IL)-15 and interferon (IFN)γ in the outer root sheath of the hair follicle. Upregulation of IL-15, NKG2D ligands, and major histocompatibility complex (MHC) class 1 molecules leads to a collapse of the immune privilege, and thus ultimately to AA (see Figure) [2-4].
AA is sometimes triggered by viral infections, such as influenza, which cause excess production of IFNγ. IFNγ is certainly one of the key factors that lead to the collapse of immune privilege [4].
Figure: Induction of alopecia areata. Adapted from [1,2]
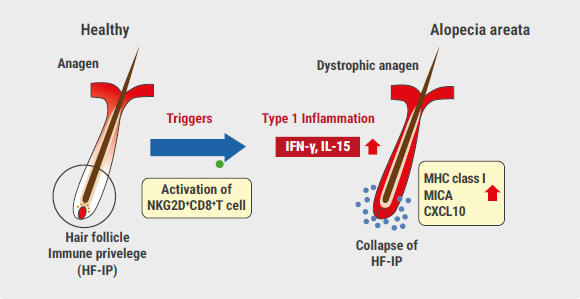 HF-IP, hair follicle immune privilege; IFN, interferon; IL, interleukin; MHC, major histocompatibility complex.
HF-IP, hair follicle immune privilege; IFN, interferon; IL, interleukin; MHC, major histocompatibility complex.Why JAK-inhibitors?
A possible therapeutic approach for AA is the restoration of this immune privilege by reducing CD8+ T lymphocytes and IL-15. JAK-1/3 signalling mediates IL-15 activation of T lymphocytes [5]. IL-15 is highly expressed in human and murine AA. It drives CD8 killer activation. JAK inhibitors block both IL-15 and IFNγ.
A breakthrough in AA therapy was a study published in 2014 in which the oral JAK inhibitor tofacitinib reversed alopecia universalis in a 25-year-old patient with plaque psoriasis [6]. Complete regrowth of hair was achieved after 8 months. Significant hair growth was also achieved in another case report with tofacitinib treatment [7]. In 2017, a study investigating AA treatment with oral tofacitinib found that 7 out of 13 participants (53.8%) achieved hair regrowth of at least 50% independent of age, disease severity, and disease duration with minimal side effects [8]. Efficacy of tofacitinib was even shown in longstanding AA: After 5 years of previous treatment with little or no response, a patient achieved total hair regrowth after therapy with tofacitinib (5 mg twice daily for a year) [9]. “However, treatment with tofacitinib is not always effective and there is a relapse after initial success in some patients,” said Prof. Ito.
AA: Also type 2 inflammation?
A study published in 2018 showed dual efficacy of the IL-4/IL-13 blocker dupilumab in a patient with atopic dermatitis and concomitant AA [10]. Due to severe, refractory AD, a young man was treated with dupilumab. In addition to response to dupilumab, the patient experienced hair regrowth starting at month 3 with almost full recovery at month 6.
JAK inhibitors show effectiveness for both AA and atopic dermatitis and are therefore promising candidates for future therapy of AA. At the moment, many JAK inhibitors are in the clinical development stage for AA.
- Ito T. JAK family inhibitors – In alopecia areata. D1T06.3B, EADV 2020 Virtual Congress, 29-31 Oct.
- Ito T, et al. Am J Pathol 2004;164:623-34.
- Paus R, et al. J Investig Dermatol Symp Proceed 2018:19:S12-17.
- Ito T. Clin Dev Immunol 2013;2013:348546.
- Ghoreschi K, et al. J Immunol 2011;186:4234–43.
- Craiglow BG, King BA. J Invest Dermatol 2014;134:2988-90.
- Jabbari A, et al. Exp Dermatol 2016:25:642-3.
- Ibrahim O, et al. JAMA Dermatol 2017;153:600-602.
- Ferreira RB, et al. Clin Case Rep 2019;7:2539-42.
- Darrigade A-S, et al. Br J Dermatol 2018;179:534-6.
Posted on
Previous Article
« Chronic spontaneous urticaria: hives, wheals & biomarkers Next Article
Borreliosis: A multifaceted disease »
« Chronic spontaneous urticaria: hives, wheals & biomarkers Next Article
Borreliosis: A multifaceted disease »
Table of Contents: EADV 2020
Featured articles
Late-Breaking News
Selective IL-23 blocker shows potential in psoriasis treatment
Promising results with nanobody treatment in psoriasis
Light at the end of the tunnel for chronic hand eczema
Epidermolysis bullosa: Novel wound treatment on the horizon
Efficacious non-steroidal topical for psoriasis
Oral JAK 1 inhibitor leads to fast itch relieve and remarkable skin clearance in AD
COVID-19: What Dermatologists Need to Know
Biologic psoriasis treatment and COVID-19 risk: Contradictory results
Much to be learned about COVID-19 and the skin
JAK Inhibitors – A Fascinating Novel Drug Class
JAK inhibitors in AA: re-establishing the immune privilege of hair follicles
JAK1 inhibition successful in hidradenitis suppurativa
Topical JAK inhibition: a novel treatment option for patients with mild-to-moderate AD
Urticaria – What’s new
Chronic inducible urticaria can require some detective work
Chronic spontaneous urticaria: hives, wheals & biomarkers
Ligelizumab for chronic spontaneous urticaria: a new star on the horizon
Infectious Diseases: Novel Developments
Bacterial resistance in skin infections – a challenging threat
Borreliosis: A multifaceted disease
Scabies – A global health challenge
Upcoming Treatments
Meaningful sleep improvement with IL-13 inhibition
Preventing foot odour with zinc oxide coated socks
Baricitinib in AD: Efficacy paired with consistent long-term results
Best of the Posters
Real-world data on brodalumab affirms efficacy and fast onset of action
Heightened risk for psychiatric comorbidities in hidradenitis suppurativa patients
Effects IL-13 blocker improves with longer treatment duration
Related Articles
December 17, 2020
Light at the end of the tunnel for chronic hand eczema
December 17, 2020
Risky sexual behaviour and STIs on the rise despite the pandemic
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

