The JAK inhibitor upadacitinib was specifically developed for therapy of AD because key cytokines involved in the pathogenesis of AD signal via the JAK1 pathway [2,3]. A previous phase 2b trial demonstrated that upadacitinib monotherapy was efficacious with a favourable benefit-risk profile compared with placebo in adults with moderate-to-severe AD. The 2 replicate, randomised, double-blind, phase 3 trials Measure Up 1 (NCT03569293) and Measure Up 2 (NCT03607422) aimed to evaluate the efficacy and safety of monotherapy with upadacitinib versus placebo in adolescents and adults with moderate-to-severe AD [1].
Prof. Emma Guttman-Yassky (Icahn School of Medicine at Mount Sinai, USA) presented the results of both trials [1]. Participants were 12-75 years old and had AD symptoms for at least 3 years. Their Eczema Area and Severity Index (EASI) was ≥16 and they had a worst pruritus score on a numerical rating scale (NRS) of ≥4. In the double-blind treatment phase, participants were randomised to upadacitinib in 2 doses (i.e. 15 mg or 30 mg once daily) or placebo. Co-primary endpoints of the trials were a ≥75% reduction in EASI and a validated Investigator´s Global Assessment of 0 or 1 (vIGA-AD 0/1; i.e. clear or almost clear skin) with ≥2 grades of reduction.
Data from 847 participants in Measure Up 1 and 836 participants in Measure Up 2 were analysed at the end of the double-blind phase. At week 16, significantly more patients treated with upadacitinib in the Measure Up 1 and 2 studies achieved the co-primary endpoints EASI 75 and vIGA-AD 0/1 (P<0.001 for all doses and endpoints; see Figure). In addition, both studies met all ranked secondary endpoints. The higher dose of upadacitinib achieved numerically better results, but both doses were significantly superior to placebo. “An EASI 100 response was achieved in 27% of patients treated with the high dose,” said Prof. Guttman-Yassky. Regarding the co-primary endpoints, a noticeable difference was already seen at week 1, which reached a plateau at week 4.
Figure: Co-primary endpoints EASI 75 and validated IGA-AD 0/1 at week 16 [1]
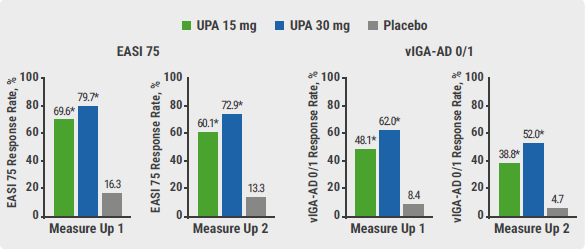 * P≤0.001 vs placebo (multiplicity controlled).
* P≤0.001 vs placebo (multiplicity controlled).EASI, Eczema Area and Severity Index; UPA, upadacitinib; PBO, placebo; vlGA-AD, validated Investigator’s Global Assessment for atopic dermatitis.
Really early significant improvement in worst pruritus NRS was already seen at days 2 and 3. The proportion of patients achieving a clinically meaningful itch reduction was significantly higher than placebo from day 2 on. The effect reached a plateau at week 4 and was maintained through week 16.
Concerning safety, acne was the most frequently reported treatment-related adverse event, reported in 19 patients treated with the low dose and 49 patients with the high dose, but only 2 patients discontinued treatment due to acne. “We saw some eczema herpeticum in the higher dose group, but no patient discontinued,” added Prof. Guttman. No new safety signals and no death were reported in the treatment arms. “The speed and the magnitude of response are really characteristic features of upadacitinib,” Prof. Gutmann-Yassky concluded.
- Guttman-Yassky E, et al. Safety and efficacy of upadacitinib monotherapy in adolescents and adults with moderate-to-severe atopic dermatitis: Results from 2 pivotal, phase 3, randomized, double-blinded, monotherapy, placebo-controlled studies (Measure Up 1 and Measure Up 2). Late-Breaker D3T03.3B, EADV 2020 Virtual Congress, 29-31 Oct.
- Parmentier JM, et al. BMC Rheumatol 2018;2:23.
- Nader A, et al. J Clin Pharmacol 2019;60:528-39.
Posted on
Previous Article
« Interview past EADV President Prof. Carle Paul Next Article
Efficacious non-steroidal topical for psoriasis »
« Interview past EADV President Prof. Carle Paul Next Article
Efficacious non-steroidal topical for psoriasis »
Table of Contents: EADV 2020
Featured articles
Late-Breaking News
Selective IL-23 blocker shows potential in psoriasis treatment
Promising results with nanobody treatment in psoriasis
Light at the end of the tunnel for chronic hand eczema
Epidermolysis bullosa: Novel wound treatment on the horizon
Efficacious non-steroidal topical for psoriasis
Oral JAK 1 inhibitor leads to fast itch relieve and remarkable skin clearance in AD
COVID-19: What Dermatologists Need to Know
Biologic psoriasis treatment and COVID-19 risk: Contradictory results
Much to be learned about COVID-19 and the skin
JAK Inhibitors – A Fascinating Novel Drug Class
JAK inhibitors in AA: re-establishing the immune privilege of hair follicles
JAK1 inhibition successful in hidradenitis suppurativa
Topical JAK inhibition: a novel treatment option for patients with mild-to-moderate AD
Urticaria – What’s new
Chronic inducible urticaria can require some detective work
Chronic spontaneous urticaria: hives, wheals & biomarkers
Ligelizumab for chronic spontaneous urticaria: a new star on the horizon
Infectious Diseases: Novel Developments
Bacterial resistance in skin infections – a challenging threat
Borreliosis: A multifaceted disease
Scabies – A global health challenge
Upcoming Treatments
Meaningful sleep improvement with IL-13 inhibition
Preventing foot odour with zinc oxide coated socks
Baricitinib in AD: Efficacy paired with consistent long-term results
Best of the Posters
Real-world data on brodalumab affirms efficacy and fast onset of action
Heightened risk for psychiatric comorbidities in hidradenitis suppurativa patients
Effects IL-13 blocker improves with longer treatment duration
Related Articles
December 17, 2020
Scabies – A global health challenge
December 17, 2020
Epidermolysis bullosa: Novel wound treatment on the horizon
December 17, 2020
Real-world data on brodalumab affirms efficacy and fast onset of action
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

