Hand eczema is characterised by painful, pruritic, non-infectious inflammatory skin changes on the hands and wrist. The disease is considered chronic when it lasts ≥3 months or relapses at least twice a year. Current topical treatment options are limited to emollients, topical corticosteroids, and calcineurin inhibitors.
The novel, topical pan-JAK inhibitor delgocitinib prevents inflammation by blocking several cytokine-mediated signalling cascades involved in the pathophysiology of chronic hand eczema. This explains the motivation for a recent phase 2b, dose-finding study in chronic hand eczema patients assessing the safety and efficacy of this agent [1]. Participants (n=258) were randomised to delgocitinib cream in 4 different doses (i.e. 1 mg/g, 3 mg/g, 8 mg/g, and 20 mg/g) or a vehicle cream. All treatments were applied twice daily. The participants had mild-to-severe chronic hand eczema (Investigator´s Global Assessment [IGA] ≥2) and had shown an inadequate response to topical corticosteroids within 1 year before screening. “Most of the 258 patients had moderate chronic hand eczema, reflected by a Hand Eczema Severity Index [HECSI] score of 44.5,” Prof. Margitta Worm (Charité – Universitätsmedizin Berlin, Germany) explained during the presentation of the study. The primary study endpoint was an IGA for chronic hand eczema (IGA-CHE) score of 0 (i.e. clear) or 1 (i.e. almost clear) with at least a 2-step improvement from baseline to week 16. A key secondary endpoint was a change in HECSI from baseline to week 16.
A statistically significant response was observed in the primary endpoint for participants receiving delgocitinib 8 mg/g and 20 mg/g (P<0.0004 vs vehicle). This significant effect was consistently demonstrated from week 4 for the 8 mg/g delgocitinib group and week 6 for the 20 mg/g delgocitinib group. IGA-CHE treatment success was achieved by 37.7% of patients treated with 20 mg/g delgocitinib and 36.5% of patients treated with 8 mg/g delgocitinib.
In addition, a statistically significant dose-response was established for the change in HECSI from baseline to week 16; all active arms had a significantly greater change in HECSI from baseline to week 16 than vehicle (P<0.05), which was most pronounced in the 2 highest doses. A significant treatment effect of delgocitinib 8 mg/g and 20 mg/g was already noticed at week 2 (P<0.05). Delgocitinib also showed a favourable safety profile. The majority of adverse events were non-serious, mild, or moderate, and not considered treatment related. The most frequently reported adverse events were nasopharyngitis, eczema, and headache.
As Prof. Worm mentioned in the discussion, the number of patients in the different treatment groups was too small to discriminate between different subgroups of hand eczema and their response. “We have to wait for phase 3 data, but delgocitinib is definitely interesting in a disease where we have hardly any options,” Prof. Worm concluded.
Oral JAK inhibitor achieves fast reduction of target lesions by almost 70%
A second phase 2b study (NCT03728504) in patients with chronic hand eczema assessed the oral JAK inhibitor gusacitinib [2]. This agent has a broad spectrum of action blocking JAK1, JAK2, JAK3, TYK2, and SYK. Thus, gusacitinib targets both the Th1 and Th2 cytokine pathways, the Th17 and Th22 cytokine pathways as well as the SYK-mediated IL-17 signalling in keratinocytes.
Prof. Howard Sofen (University of California, Los Angeles, USA) presented the results. All study participants (n=97) had moderate-to-severe chronic hand eczema and were refractory to corticosteroids (locally and/or systemic). The participants were randomised to gusacitinib 40 mg, gusacitinib 80 mg, or placebo. At 16 weeks, placebo-treated patients could switch to the higher dose of gusacitinib for continued treatment. The primary endpoint was the percentage change in the modified Total Lesion Symptom Score (mTLSS) at week 16.
Gusacitinib in the higher dose reduced the mTLSS by almost 70% (see Figure). A significant difference with placebo was seen as early as after 2 weeks. The lower dose was only significantly more effective than placebo at 2 weeks. At this time, almost a third of patients treated with the higher dose achieved the secondary endpoint of Physician Global Assessment (PGA) 0-1 (i.e. clear/almost clear). “31.3% achieved a minimal disease activity, a 5-fold difference in response compared with placebo,” explained Prof. Sofen. In addition, gusacitinib provided a rapid and significant improvement in pruritus.
Adverse events evaluated were mostly sporadic and mild or moderate in severity. No significant changes were seen in haematology lab values or changes in total cholesterol, no opportunistic infections, thromboembolic events or major adverse cardiovascular event, malignancies, or deaths.
Figure: Percentage reduction in mTLSS at week 16 (primary endpoint) [1]
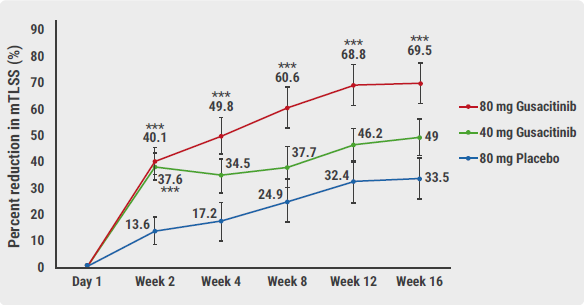 mTLSS, modified Total Lesion Symptom Score.
mTLSS, modified Total Lesion Symptom Score.*P<0.05; **P<0.01; ***P<0.005.
- Worm M, et al. The topical pan-JAK inhibitor delgocitinib cream demonstrates dose response in a 16-week phase 2b trial in chronic hand eczema. D1T03.4A, EADV 2020 Virtual Congress, 29-31 Oct.
- Sofen H, et al. A Phase 2b, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy, Safety, Tolerability, and Pharmacokinetics of Gusacitinib (ASN002) in Subjects with Moderate-to-Severe Chronic Hand Eczema. D1T03.4C, EADV 2020 Virtual Congress, 29-31 Oct.
Posted on
Previous Article
« Epidermolysis bullosa: Novel wound treatment on the horizon Next Article
Promising results with nanobody treatment in psoriasis »
« Epidermolysis bullosa: Novel wound treatment on the horizon Next Article
Promising results with nanobody treatment in psoriasis »
Table of Contents: EADV 2020
Featured articles
Late-Breaking News
Selective IL-23 blocker shows potential in psoriasis treatment
Promising results with nanobody treatment in psoriasis
Light at the end of the tunnel for chronic hand eczema
Epidermolysis bullosa: Novel wound treatment on the horizon
Efficacious non-steroidal topical for psoriasis
Oral JAK 1 inhibitor leads to fast itch relieve and remarkable skin clearance in AD
COVID-19: What Dermatologists Need to Know
Biologic psoriasis treatment and COVID-19 risk: Contradictory results
Much to be learned about COVID-19 and the skin
JAK Inhibitors – A Fascinating Novel Drug Class
JAK inhibitors in AA: re-establishing the immune privilege of hair follicles
JAK1 inhibition successful in hidradenitis suppurativa
Topical JAK inhibition: a novel treatment option for patients with mild-to-moderate AD
Urticaria – What’s new
Chronic inducible urticaria can require some detective work
Chronic spontaneous urticaria: hives, wheals & biomarkers
Ligelizumab for chronic spontaneous urticaria: a new star on the horizon
Infectious Diseases: Novel Developments
Bacterial resistance in skin infections – a challenging threat
Borreliosis: A multifaceted disease
Scabies – A global health challenge
Upcoming Treatments
Meaningful sleep improvement with IL-13 inhibition
Preventing foot odour with zinc oxide coated socks
Baricitinib in AD: Efficacy paired with consistent long-term results
Best of the Posters
Real-world data on brodalumab affirms efficacy and fast onset of action
Heightened risk for psychiatric comorbidities in hidradenitis suppurativa patients
Effects IL-13 blocker improves with longer treatment duration
Related Articles

December 18, 2020
Letter from the Editor
December 17, 2020
Risky sexual behaviour and STIs on the rise despite the pandemic
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

