Chronic inducible urticaria (CindU) is less common than spontaneous urticaria but can also have a dramatic negative impact on quality of life. Novel treatment options aim at depleting or silencing mast cells [1].
“About 1 in 4 patients suffers from CindU, the most common being symptomatic dermographism, cold urticaria, and cholinergic urticaria,” Prof. Marcus Maurer (Charité Universitätsmedizin Berlin, Germany) explained [1]. COLD-CE is an international, multicentre, prospective, cross-sectional academic study aiming to better understand cold-induced urticaria. In this study, real-world data is collected on still ill-defined characteristics, such as subforms of cold-induced urticaria. The researchers found subforms like food-dependent CindU. One patient only suffered from cold-induced urticaria after a specific meal: steak. “There are rare subforms that require our detective work and these projects are in the process of identifying and characterising them,” Prof. Maurer said.
Regarding therapy, omalizumab seems to be effective in cold urticaria, although it is not an approved indication. At the moment, many different biologics are developed for CindU and other forms of urticaria. An interesting novel approach is CDX-0159, an anti-KIT monoclonal antibody that aims to starve mast cells by inhibiting differentiation, migration, and maturation of mast cells (see Figure).
Figure: CDX-0159, an anti-KIT monoclonal antibody, aims to treat CindU by mast cell depletion [2]
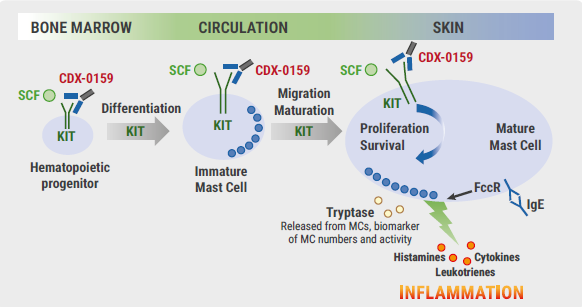
CindU, chronic inducible urticaria; IgE, Immunoglobulin E; MC, mast cell; SCF, stem cell factor
Cholinergic urticaria is brought on by raised body temperature or sweating and entails a significant burden for affected patients. “We put patients on a treadmill and challenge them because then we can determine thresholds before wheals start to come,” Prof. Maurer explained. This can greatly help patients to cope with the situation. Antihistamine treatment is often ineffective in cholinergic urticaria. Sometimes, a response can be gained by updosing but a little more than 60% of patients with cholinergic urticaria do not respond sufficiently to elevating antihistamines. “So, clearly, we have to develop better treatment options,” stated Prof. Maurer. A complete response to omalizumab standard dose was shown by 13% of patients, and 47% had at least a major response. “If you change the dose and interval of omalizumab, you get up to two-thirds of patients to respond,” Prof. Maurer recommended. However, even with optimised dosing, there are non-responders, particularly in the male population. Furthermore, the standard dose is often not sufficient in patients with cholinergic urticaria.
Another novel approach is the silencing of mast cells with Siglec-8, an inhibitory mast cell silencing receptor antibody. In a phase 1b study, 7 patients that were treated with this agent showed a very good response. In addition, there is an ongoing study with dupilumab, the CHED study (NCT NCT03749148), in patients with cholinergic urticaria.
There are also new tools to assess disease activity and disease control in CindU, the most important being the urticaria control test. “Let’s use these tools in clinical practice to better treat patients,” Prof. Maurer concluded.
- Maurer M. News in inducible urticarias. D2T05.1C, EADV 2020 Virtual Congress, 29-31 Oct.
- Maurer M, et al. CDX-0159, an anti-KIT monoclonal antibody, demonstrates dose-dependent reductions in serum tryptase and a favorable safety profile in a phase 1a healthy volunteer study. Abstract 1829, EAACI Digital Congress 2020, 6-8 June.
Posted on
« Borreliosis: A multifaceted disease Next Article
Bacterial resistance in skin infections – a challenging threat »
Table of Contents: EADV 2020
Featured articles
Late-Breaking News
Selective IL-23 blocker shows potential in psoriasis treatment
Promising results with nanobody treatment in psoriasis
Light at the end of the tunnel for chronic hand eczema
Epidermolysis bullosa: Novel wound treatment on the horizon
Efficacious non-steroidal topical for psoriasis
Oral JAK 1 inhibitor leads to fast itch relieve and remarkable skin clearance in AD
COVID-19: What Dermatologists Need to Know
Biologic psoriasis treatment and COVID-19 risk: Contradictory results
Much to be learned about COVID-19 and the skin
JAK Inhibitors – A Fascinating Novel Drug Class
JAK inhibitors in AA: re-establishing the immune privilege of hair follicles
JAK1 inhibition successful in hidradenitis suppurativa
Topical JAK inhibition: a novel treatment option for patients with mild-to-moderate AD
Urticaria – What’s new
Chronic inducible urticaria can require some detective work
Chronic spontaneous urticaria: hives, wheals & biomarkers
Ligelizumab for chronic spontaneous urticaria: a new star on the horizon
Infectious Diseases: Novel Developments
Bacterial resistance in skin infections – a challenging threat
Borreliosis: A multifaceted disease
Scabies – A global health challenge
Upcoming Treatments
Meaningful sleep improvement with IL-13 inhibition
Preventing foot odour with zinc oxide coated socks
Baricitinib in AD: Efficacy paired with consistent long-term results
Best of the Posters
Real-world data on brodalumab affirms efficacy and fast onset of action
Heightened risk for psychiatric comorbidities in hidradenitis suppurativa patients
Effects IL-13 blocker improves with longer treatment duration
Related Articles

Letter from the Editor
Chronic inducible urticaria can require some detective work
Chronic spontaneous urticaria: hives, wheals & biomarkers
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

