In the pivotal phase 3 ECZTRA 1 (NCT03131648) and ECZTRA 2 (NCT03160885) trials in adults with moderate-to-severe AD, tralokinumab monotherapy provided significant and early improvements in clinically relevant endpoints. In both trials, significantly more patients receiving tralokinumab monotherapy than placebo achieved the primary endpoints of Investigator’s Global Assessment (IGA) 0/1, equivalent to clear or almost clear skin, and 75% improvement in Eczema Area and Severity Index (EASI 75). However, these are stringent endpoints; hence, achievement of mild disease severity (IGA 2) and a 50% improvement in EASI could already be considered clinically relevant for most patients. The current post-hoc analysis evaluated the treatment results of patients who did not achieve the primary endpoint at week 16 and continued to receive open-label tralokinumab plus optional topical corticosteroids (TCS) for an additional 36 weeks [1].
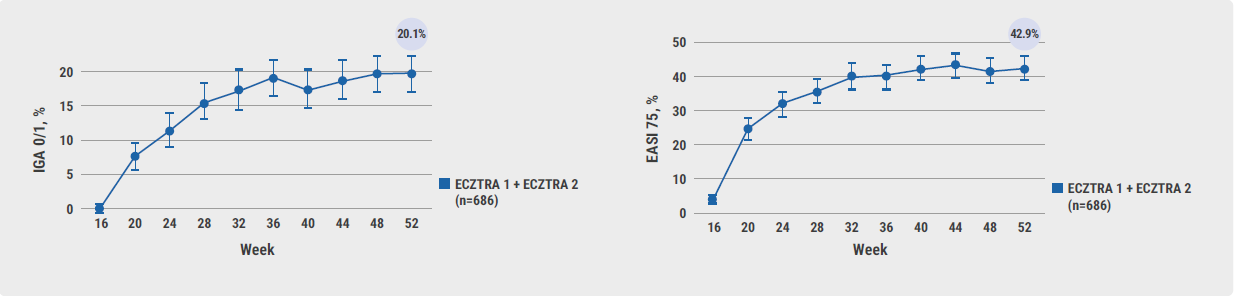
After 52 weeks, 20.1% of patients treated with tralokinumab plus optional TCS achieved an IGA 0/1 response, and 42.9% achieved an EASI 75 response (see Figure). More than half of the responder proportions at week 52 were achieved within 8 weeks of starting open-label treatment.
Figure: Participants achieving (A) IGA 0/1 and (B) EASI 75 at week 52 in the open-label phase [1]
Late response not due to TCS therapy
To determine whether the improved response over time was due to TCS or tralokinumab, another analysis was performed in which participants who used concomitant anti-inflammatory treatment (49.3%) were considered non-responders. In this alternative analysis, the response rates were 13.9% and 25.7% for IGA 0/1 and EASI 75, respectively, at week 52 without TCS.
The authors concluded that adult patients who did not achieve IGA 0/1 or EASI 75 at week 16 progressively improved with continued tralokinumab treatment beyond week 16. The clinical response with continued treatment beyond week 16 was mainly driven by continued tralokinumab treatment and not by the addition of optional TCS.
- Simpson E, et al. Tralokinumab provides progressive improvements beyond week 16 in patients with atopic dermatitis with an initial partial response. Poster P0214, EADV 2020 Virtual Congress, 29-31 Oct.
Posted on
Previous Article
« MRI-based nomograms may predict lymph node metastasis, survival in early breast cancer Next Article
Interview past EADV President Prof. Carle Paul »
« MRI-based nomograms may predict lymph node metastasis, survival in early breast cancer Next Article
Interview past EADV President Prof. Carle Paul »
Table of Contents: EADV 2020
Featured articles
Late-Breaking News
Selective IL-23 blocker shows potential in psoriasis treatment
Promising results with nanobody treatment in psoriasis
Light at the end of the tunnel for chronic hand eczema
Epidermolysis bullosa: Novel wound treatment on the horizon
Efficacious non-steroidal topical for psoriasis
Oral JAK 1 inhibitor leads to fast itch relieve and remarkable skin clearance in AD
COVID-19: What Dermatologists Need to Know
Biologic psoriasis treatment and COVID-19 risk: Contradictory results
Much to be learned about COVID-19 and the skin
JAK Inhibitors – A Fascinating Novel Drug Class
JAK inhibitors in AA: re-establishing the immune privilege of hair follicles
JAK1 inhibition successful in hidradenitis suppurativa
Topical JAK inhibition: a novel treatment option for patients with mild-to-moderate AD
Urticaria – What’s new
Chronic inducible urticaria can require some detective work
Chronic spontaneous urticaria: hives, wheals & biomarkers
Ligelizumab for chronic spontaneous urticaria: a new star on the horizon
Infectious Diseases: Novel Developments
Bacterial resistance in skin infections – a challenging threat
Borreliosis: A multifaceted disease
Scabies – A global health challenge
Upcoming Treatments
Meaningful sleep improvement with IL-13 inhibition
Preventing foot odour with zinc oxide coated socks
Baricitinib in AD: Efficacy paired with consistent long-term results
Best of the Posters
Real-world data on brodalumab affirms efficacy and fast onset of action
Heightened risk for psychiatric comorbidities in hidradenitis suppurativa patients
Effects IL-13 blocker improves with longer treatment duration
Related Articles
December 17, 2020
Borreliosis: A multifaceted disease
December 17, 2020
Chronic spontaneous urticaria: hives, wheals & biomarkers
December 17, 2020
Biologic psoriasis treatment and COVID-19 risk: Contradictory results
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

