Vedolizumab in anti-TNF-naïve IBD patients
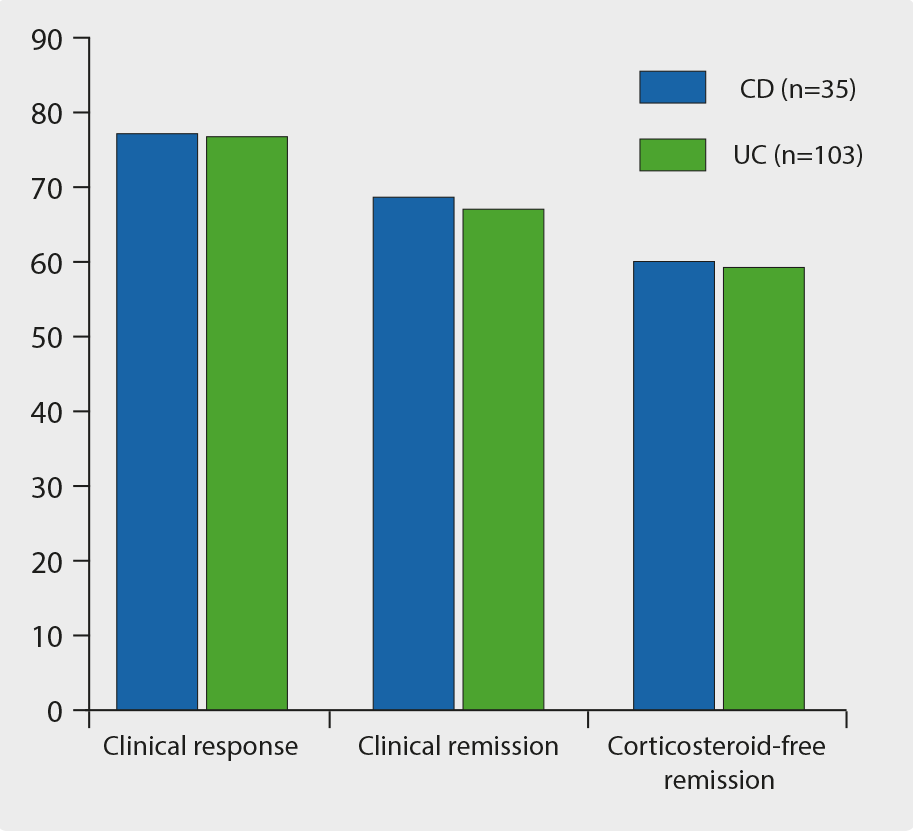
The effectiveness and safety of vedolizumab in anti-TNF-naïve patients was demonstrated in a real-world setting using a retrospective, multicentre, pooled cohort study [1]. This included consecutive, active, anti-TNF-naïve IBD patients treated with vedolizumab. The primary outcome was clinical response at week 14. Patients with follow-up beyond week 14 and those discontinuing vedolizumab at any time were included for last follow-up analysis. Since January 2015, anti-TNF-naïve patients (CD n=50; UC n=134) initiated vedolizumab treatment. In CD, 82% of patients responded by week 14, and 64% were in clinical remission. A total of 52% achieved corticosteroid-free remission. At last follow-up (44 weeks), 77.1% of patients with available data had responded to treatment: 68.6% were in clinical remission, 60% in corticosteroid-free remission. In UC, 79.1% of patients responded to treatment and 39.5% were in remission by week 14; 36.6% of patients achieved corticosteroid-free remission. At the last follow-up (42.5 weeks), 76.7% of patients had responded to treatment: 67.0% were in remission, and 59.2% in corticosteroid-free remission (see Figure).
Figure: Efficacy of vedolizumab in CD and UC at last follow-up [1]
Endoscopic improvement was achieved in 63.7% and mucosal healing in 45.5% of CD patients with available data from baseline till follow-up. In UC, mucosal healing was achieved in 58.5% of patients. Secondary loss of response developed after 39 weeks of follow-up in 10% of the patients who continued treatment at week 14. AEs were reported in 11% of the patients; in 3.3%, this lead to treatment discontinuation.
It was concluded that vedolizumab was effective in inducing clinical remission and response in anti-TNF-naïve patients, with similar response rates in UC and CD. The efficacy of vedolizumab is higher than has been reported in anti-TNF-experienced patients and is comparable to that of anti-TNF biologics in this population.
Vedolizumab shows long-term effectiveness and favourable safety profile
A post-hoc analysis was done of 421 vedolizumab-naïve patients from the GEMINI long-term safety studies [2]. This showed that almost two-thirds of UC patients and more than half of CD patients persisted with vedolizumab treatment for three years.
Rates of vedolizumab discontinuation due to AEs were low and vedolizumab treatment persistence rates were higher in patients without prior TNF antagonist failure. Vermeire et al. used this de novo cohort from the GEMINI study to validate the long-term effectiveness and favourable safety profile of vedolizumab. The cohort consisted of a population without prior vedolizumab exposure.
These patients were more likely to represent patients physicians see in their daily clinical practice than patients who had participated in randomised controlled trials. These de novo patients (n=421; UC n=190, CD n=231) received vedolizumab every four weeks. Among them, 61% of UC patients and 74% of CD patients had prior TNF antagonist failure. Median disease duration was 5.8 years for UC and 8.3 years for CD, respectively. The number of patients who discontinued vedolizumab treatment during follow-up was 218, the main reason being lack of efficacy in 46% and AEs in 27%.
The respective survival probabilities of continuing vedolizumab at one and three years were 77% and 64% in UC patients, and 67% and 55% in CD patients. These probabilities were higher in patients without than in patients with prior TNF antagonist failure. For UC patients, they were 81% vs. 75% at one year, 69% vs. 61% at three years (P=0.18). For CD patients, the values were 84% vs. 60% at one year, 68% vs. 51% at three years (P<0.01). Cumulative probabilities of treatment discontinuation for lack of efficacy at one and three years were: 16% and 22% in UC, and 26% and 33% in CD. For AEs, this was 8% and 17% in UC, and 10% and 18% in CD.
These data were concluded to support the long-term effectiveness and favourable safety profile of vedolizumab and suggest improved outcomes of vedolizumab treatment in TNF antagonist-naïve patients.
- Kopylov U, et al. DOP001. ECCO 2018.
- Vermeire S, et al. DOP002. ECCO 2018.
Posted on
Previous Article
« ILC1 distribution predicts response to ustekinumab Next Article
Long-term risk of advanced neoplasia after colonic low-grade dysplasia in IBD »
« ILC1 distribution predicts response to ustekinumab Next Article
Long-term risk of advanced neoplasia after colonic low-grade dysplasia in IBD »
Table of Contents: ECCO 2018
Featured articles
IBD diagnostics
IBD disease patterns and genetics
Novel treatment strategies
Efficacy and safety of biologics
Oncology in IBD
Surgery for IBD
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy