Apremilast is an oral small-molecule inhibitor of phosphodiesterase-4 which modulates a wide array of pro- and anti-inflammatory mediators in UC. Application showed clinically meaningful improvement in symptoms, endoscopy, biomarkers, and mucosal healing compared with placebo.
A phase-2 study enrolled 170 patients who failed at least one conventional UC therapy and who were naïve to biologic therapy. These patients had active UC, defined as a total Mayo score (TMS) of 6 to 11, with a Mayo endoscopic score (MES) ≥2). They were randomised 1:1:1 to receive apremilast 30 mg twice daily (BID; n=57), apremilast 40 mg BID (n=55), or placebo (n=58) for up to 12 weeks. Mean baseline TMS and MES were 8.3 and 2.6, respectively.
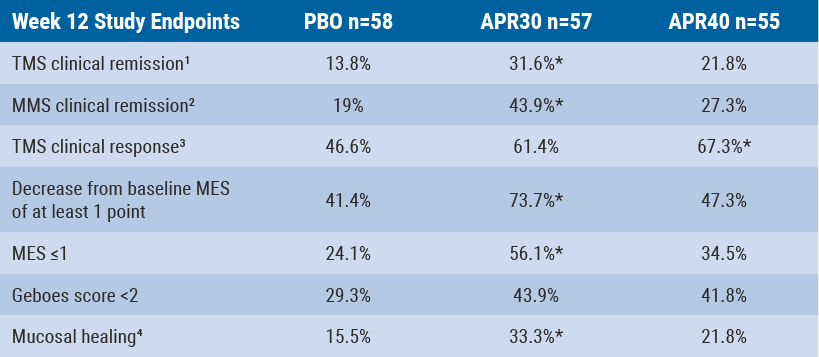
At week 12, significantly more patients treated with apremilast 30 mg than placebo achieved TMS clinical remission as well as modified Mayo score clinical remission. A higher proportion of patients treated with apremilast 30 mg and apremilast 40 mg achieved a TMS clinical response compared with placebo.
However, the result was only significant for the apremilast 40 mg treatment group. Significantly more patients treated with apremilast 30 mg than placebo achieved a decrease of at least one point from baseline MES and an MES ≤1. Compared with placebo, both apremilast treatment groups showed a trend for a higher proportion of patients achieving histological remission, defined as a Geboes score <2. A significantly higher proportion of patients treated with apremilast 30 mg than with placebo achieved mucosal healing (MES ≤1 with Geboes score <2) (see Table).
Table: Results of the study endpoints at week 12[9]
Patients treated with apremilast 30 mg achieved significant improvements in amount of change from baseline in high-sensitive CRP at weeks 4, 8, and 12. The same was true for FCP at weeks 2, 4, and 8 compared with placebo. A similar safety profile was seen in patients treated with apremilast 30 mg and apremilast 40 mg. Any AEs which occurred during the study were consistent with what could be expected in UC patients or the known safety profile of apremilast [9].
- Danese S, et al. OP006. ECCO 2018.
Posted on
Previous Article
« Shallow whole-genome sequencing predicts future cancer risk of LGD in UC Next Article
Significant reduction of emergency stoma surgeries for CD »
« Shallow whole-genome sequencing predicts future cancer risk of LGD in UC Next Article
Significant reduction of emergency stoma surgeries for CD »
Table of Contents: ECCO 2018
Featured articles
IBD diagnostics
IBD disease patterns and genetics
Novel treatment strategies
Efficacy and safety of biologics
Oncology in IBD
Surgery for IBD
Related Articles
May 28, 2018
Genetic predisposition for gut barrier dysfunction

May 28, 2018
Apremilast in active UC shows promising results
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

