Previously, safety and efficacy of adalimumab in UC patients had been demonstrated in the ULTRA 1 and ULTRA 2 trials. LEGACY – an on-going ten-year, post-marketing, non-interventional, multinational registry – has been designed to further assess the long-term safety and effectiveness of adalimumab as used in the daily clinical practice for adult patients with moderate-to-severe active UC, thus providing real-life data. The registry also evaluates the long-term safety of patients receiving IMM (thiopurines) without concurrent biologic.
Registry TEAEs were defined as AEs occurring from the first day in the registry for up to 70 days for adalimumab or 30 days for IMM after the last dose in the registry, or up to the cut-off date of May 31, 2017. For the adalimumab registry group, data were reported with or without concurrent IMM, and overall. AEs were recorded based on concurrent IMM use at the time of occurrence.
A total of 4,245 patients enrolled in the registry, of which 4,203 patients were evaluated (adalimumab n=1,984; IMM n=2,219). Mean age at enrolment was 44.5 years with a mean UC duration of 10.3 years. Of the patients, 54% were male and 95% were white. Overall, 137 and 135 patients in the adalimumab and IMM groups, respectively, discontinued the registry. The registry data represent 2,707.77 and 3,364.54 patient-years of exposure to adalimumab and IMM, respectively. Incidence and event rates of TEAEs are shown in the Table.
Table: Incidence and event rates of TEAEs in the LEGACY registry [3]
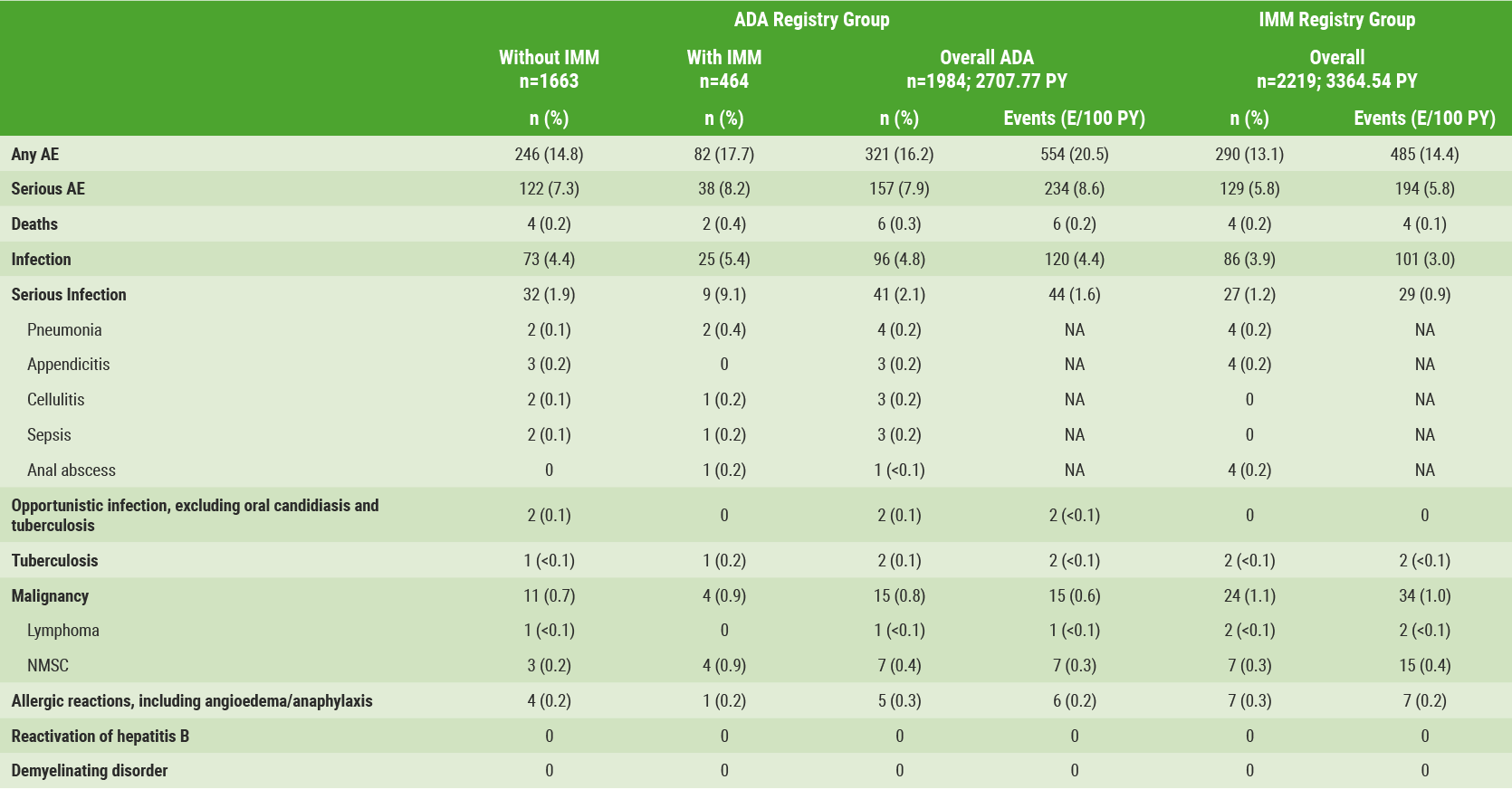
The most common serious infections in the adalimumab registry group were pneumonia (n=4), appendicitis, cellulitis and sepsis (each n=3). In the IMM registry group, anal abscess, appendicitis, and pneumonia (each n=4) predominated. Malignancies were observed in 15 patients in the adalimumab group and 24 patients in the IMM group. Deaths were recorded for six and four patients in the adalimumab and IMM registry groups, respectively. Thus, in patients with moderate-to-severe active UC, safety of adalimumab was consistent with the known safety profile; no new safety signals were identified [3].
- Bossuyt P, et al. DOP004. ECCO 2018.
Posted on
Previous Article
« Significant reduction of emergency stoma surgeries for CD Next Article
Cancer risk in IBD associated with age and recent immunomodulator use »
« Significant reduction of emergency stoma surgeries for CD Next Article
Cancer risk in IBD associated with age and recent immunomodulator use »
Table of Contents: ECCO 2018
Featured articles
IBD diagnostics
IBD disease patterns and genetics
Novel treatment strategies
Efficacy and safety of biologics
Oncology in IBD
Surgery for IBD
Related Articles
May 28, 2018
Long-term safety profile of adalimumab
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

