Vedolizumab and ustekinumab can both be considered for treatment in CD when conventional and anti-TNF medication fail.
As head-to-head trials are not available and methodological issues limit an indirect comparison based on the registration studies, Biemans et al. compared vedolizumab- and ustekinumab-treated CD patients in a real-life systematic observational cohort (Initiative on Crohn and Colitis case series) [17]. They reported that clinical remission and AEs were comparable between treatments after 24 weeks.
CD patients receiving either vedolizumab or ustekinumab in standard care were followed using predefined follow-up visits (at 0, 12, 24, 52, and 104 weeks). CD characteristics documented included clinical disease activity (HBI), inflammatory markers (CRP, FCP), hospital admissions, CD-related surgery and AEs. Clinical remission was defined as HBI <5. As the aim was to compare treatments, patients with a history of both studied treatments were excluded. Propensity score analysis was used to match, correcting for hospital of admission, gender, disease duration, location, behaviour, prior CD or perianal surgery, and number of failed biologicals. Based on similar baseline characteristics, 42 patients (out of 172) starting on vedolizumab and 42 (out of 125) starting on ustekinumab were matched (see Table).
Table: Baseline characteristics [17]
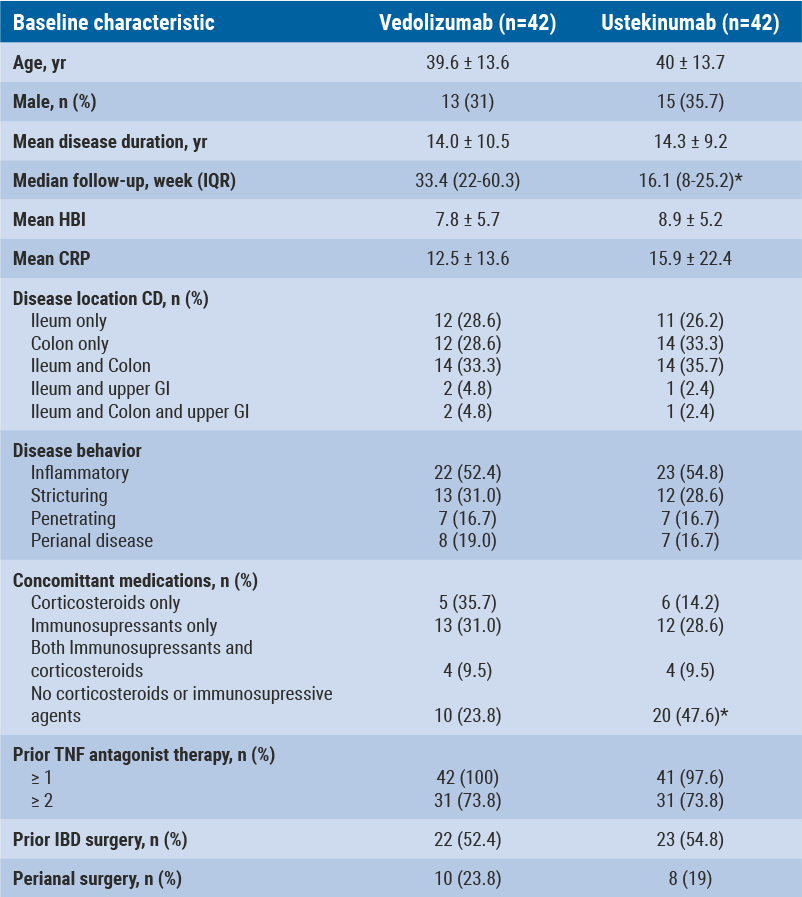
Reduction of HBI was significant for both vedolizumab and ustekinumab after 12 weeks (vedolizumab mean HBI -2.9, P=0.002; ustekinumab mean HBI -4.2, P<0.001). The mean difference in HBI decrease between vedolizumab and ustekinumab was not significant at week 12 (P=0.346). HBI did not decrease further beyond 12 weeks: at week 24 mean HBI was −3.9 on vedolizumab vs. −4.6 on ustekinumab (P=0.72). After 24 weeks, a comparable proportion of patients (46.2% vedolizumab and 57.9% ustekinumab) were in corticosteroid-free clinical remission (P=0.44). The rate of AEs was also comparable.
- Biemans V, et al. DOP052. ECCO 2018.
Posted on
Previous Article
« Diagnostic techniques for IBD: the ECCO-ESGAR Guidelines Next Article
Higher rates of remission and healing with vedolizumab vs TNF antagonist »
« Diagnostic techniques for IBD: the ECCO-ESGAR Guidelines Next Article
Higher rates of remission and healing with vedolizumab vs TNF antagonist »
Table of Contents: ECCO 2018
Featured articles
IBD diagnostics
IBD disease patterns and genetics
Novel treatment strategies
Efficacy and safety of biologics
Oncology in IBD
Surgery for IBD
Related Articles
May 28, 2018
Genetic predisposition for gut barrier dysfunction

May 28, 2018
Letter from The Editor
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

