Vedolizumab – a gut-selective humanised IgG1 monoclonal antibody – has demonstrated statistically significant differences in clinical remission for patients with moderate-to-severe active CD compared with placebo [11]. However, endoscopic healing has not been previously assessed.
Danese et al. [12] demonstrated positive effects of vedolizumab on endoscopic remission and healing in CD patients in the phase-3b, single-arm, multicentre VERSIFY study. The subjects were 101 patients with moderate-to-severe active CD (≥3 months; CDAI 220-450; SES-CD ≥7; ≥1 mucosal ulceration on centrally read endoscopy). They had previously experienced treatment failure with corticosteroids, IMM, and/or at least one TNF antagonist. Treatment included open-label standard induction dose of IV vedolizumab 300 mg on day 1, and at weeks 2, 6, 14, 22, up to week 26. They also underwent screening colonoscopy at weeks 14 and 26 with a central reader performing the endoscopic evaluations.
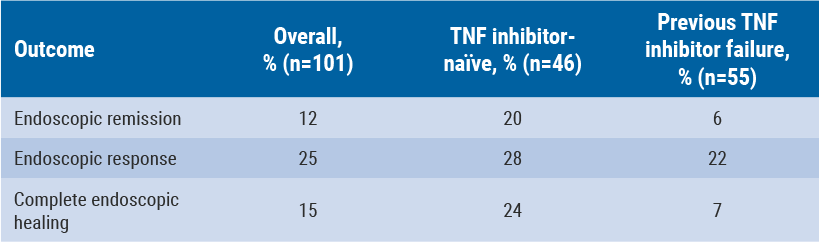
Baseline SES-CD score was 16 (endoscopic remission was defined as a score ≤4) and mean CDAI score was 324. Of these patients, 55% had failed previous treatment with a TNF inhibitor, while 40% had very high (>10 mg/L) levels of CRP. All patients had one or more mucosal ulcerations on ileocolonoscopy. At week 26, complete mucosal healing, defined as an absence of ulceration, was achieved by 15% of the overall study population. This was 24% of TNF inhibitor-naïve patients and 7% of patients who failed previous TNF inhibitor therapy (see Table).
Table: Endoscopic outcomes for vedolizumab at week 26, by previous TNF-therapy status [12]
At week 10, clinical remission (defined as a CDAI score ≤150) was achieved by 36% of all patients. This percentage increased to 42% at week 26. Patients who had not been previously treated with a TNF inhibitor did better than those previously treated, with 52% achieving clinical remission at week 26. Regarding safety and tolerability, no new safety signals were observed.
A total of 66 patients had AEs and 11 patients had treatment-emergent AEs (TEAEs), including two exacerbations of CD. No cases of liver injury or death were observed. These findings demonstrate the ability of vedolizumab to induce endoscopic remission and healing in a refractory population. TNF inhibitor-naïve patients are more likely to achieve endoscopic remission and healing than patients who failed previous treatment with a TNF antagonist [12].
- Sandborn WJ, et al. N Engl J Med, 2013;369:711-21.
- Danese S, et al. OP023. ECCO 2018.
Posted on
Previous Article
« SLC12A2 potential marker of dysplasia associated to inflammation in UC Next Article
Genetic predisposition for gut barrier dysfunction »
« SLC12A2 potential marker of dysplasia associated to inflammation in UC Next Article
Genetic predisposition for gut barrier dysfunction »
Table of Contents: ECCO 2018
Featured articles
IBD diagnostics
IBD disease patterns and genetics
Novel treatment strategies
Efficacy and safety of biologics
Oncology in IBD
Surgery for IBD
Related Articles
May 28, 2018
Combining new drugs with different mechanisms

© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

