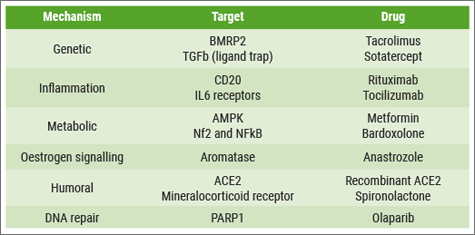
“Despite the success of these drugs in improving the wellbeing of our patients, we feel that we can do better”, said Prof. Martin Wilkins (Imperial College London, UK) at the beginning of his lecture. “The ultimate goal is to stop disease progression and, if possible, reverse the remodelling that characterises PAH. The problem is that we lack knowledge about the interplay between these pathways in the remodelling process. The good news is that we have a number of druggable targets in each of these pathogenic mechanisms (see Table). The real challenge is to prioritise and identify which of these candidate drugs might offer the most rapid progress.”
Many of these drugs have entered clinical trials. However, only prostacyclin analogues have reached the phase 3 stadium.
Table: Overview of several drug targets in PAH
Five key aspects
In selecting the optimal therapy 5 aspects are important according to Prof. Wilkins: the right target, the right tissue, the right safety, the right patient, and the right commercial potential.
“We cannot overemphasise the importance of a valid target. Selecting the right target remains the most important of the 5 aspects and the most important decision we make in drug development.”
- Humbert M et al. Circulation. 2014;130:2189-208.
- Mizoguchi H et al. Circ Cardiovasc Interv. 2012;5:748-55.
- Aoki T et al. Eur Heart J. 2017;38:3152-3159.
- Olsson KM et al. Eur Respir J. 2017;49(6).
- Ogawa A, et al. Circ J. 2018;82:1222-1230.
Posted on
Previous Article
« Letter from the Editor Next Article
Letter from the Editor »
« Letter from the Editor Next Article
Letter from the Editor »
Table of Contents: ERS 2018
Featured articles
Letter from The Editor
[Long Read] Current Look on Asthma
COPD: Triple Therapy, MABA and Antibiotics
Landmark triple therapy trials
ICS: to use or not to use?
MABA, and novel LAMA
Macrolide antibiotics and trial with azithromycin
Current Look on Asthma
[Long Read] Current Look on Asthma
Endoscopic Solutions
Endoscopic treatment of emphysema
Endoscopic treatment of asthma
Endoscopic treatment of chronic bronchitis
PAH
Balloon pulmonary angioplasty for CTEPH
New therapeutic targets: moving form pre-clinical data to phase 2 studies
IPF
Gastroesophageal reflux, IPF and lessons learned
Oncology
ALK inhibition, guidelines, liquid biopsies, and immunotherapy
Brain metastases, lung cancer and interstitial lung disease
Related Articles

October 31, 2022
Letter from the Editor
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

