The role of ICS in COPD is an area of intense interest, said Dr Gary Ferguson (Pulmonary Research Institute of Southeast Michigan, USA) at the beginning of his lecture [16]. The recently updated GOLD guidelines recommend initiation of ICS treatment for patients with high exacerbation risk only [17], due to concerns over the risk:benefit ratio of chronic ICS use compared to other therapies. However, real-world studies have shown that ICS are prescribed across the spectrum of disease severity [18].
The benefits of ICS/LAMA/LABA triple therapy are not well studied in certain patient populations, such as patients who are considered to have a lower risk of exacerbations. Budesonide/glycopyrronium/formoterol metered-dose inhaler (MDI), formulated using an innovative co-suspension delivery technology, is a fixed-dose triple combination in development for patients with COPD. “KRONOS was partly a regulatory study to evaluate triple vs dual combination therapy”, stated Dr Ferguson. In symptomatic patients with moderate-to-severe COPD, mostly GOLD B, budesonide/glycopyrronium/formoterol (320/14.4/10 μg) MDI provided clinically meaningful improvements in lung function vs budesonide/formoterol, delivered as MDI or dry powder inhalers (DPI, see Figure). This treatment effect was independent of exacerbation history.
Figure: Peak and trough FEV1 over 24 weeks in KRONOS study [16]
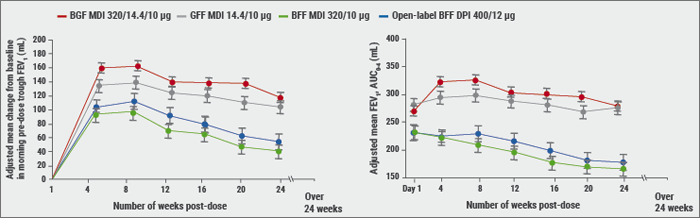
An important endpoint, according to Dr Ferguson, was the rate of moderate-to-severe COPD exacerbations over 24 weeks. Triple therapy resulted in significant, clinically meaningful reductions in exacerbation rates vs glycopyrronium/formoterol MDI, with numerical reductions vs budesonide/formoterol, delivered as MDI or DPI. There was a significant difference between triple and dual therapy with glycopyrronium/formoterol (rate ratio 0.48; 95% CI 0.37-0.64; P<0.0001). The time to first moderate-to-severe COPD exacerbation was also significantly different, again in comparison with glycopyrronium/formoterol (HR 0.593; 95% CI 0.37-0.64; P<0.0001, measured with Cox regression analysis). All treatments were well tolerated with no new or unexpected safety findings. The incidence of pneumonia was similar across treatment groups.
Favourable risk:benefit profile
According to Dr Ferguson, the favourable risk:benefit profile of budesonide/glycopyrronium/formoterol MDI challenges current recommendations for ICS use in COPD. “It raises a question of whether a broader patient population than just those with frequent exacerbations benefit from triple therapy. KRONOS supports the potential use of triple therapy in symptomatic patients with COPD who are not adequately controlled by dual therapy, irrespective of exacerbation risk.” The results of KRONOS were published online in The Lancet Respiratory Medicine [19].
- Ferguson GT, et al. Abstract OA1661, ERS 2018.
- GOLD COPD 2018, www.goldcopd.org
- Ding B, et al. Int J Chron Obstruct Pulmon Dis. 2017;12:1527-1537.
- Ferguson GT, et al. Lancet Respir Med. 2018, Sept 16.
Posted on
Previous Article
« Endoscopic treatment of chronic bronchitis Next Article
Nintedanib and sildenafil »
« Endoscopic treatment of chronic bronchitis Next Article
Nintedanib and sildenafil »
Table of Contents: ERS 2018
Featured articles
Letter from The Editor
[Long Read] Current Look on Asthma
COPD: Triple Therapy, MABA and Antibiotics
Landmark triple therapy trials
ICS: to use or not to use?
MABA, and novel LAMA
Macrolide antibiotics and trial with azithromycin
Current Look on Asthma
[Long Read] Current Look on Asthma
Endoscopic Solutions
Endoscopic treatment of emphysema
Endoscopic treatment of asthma
Endoscopic treatment of chronic bronchitis
PAH
Balloon pulmonary angioplasty for CTEPH
New therapeutic targets: moving form pre-clinical data to phase 2 studies
IPF
Gastroesophageal reflux, IPF and lessons learned
Oncology
ALK inhibition, guidelines, liquid biopsies, and immunotherapy
Brain metastases, lung cancer and interstitial lung disease
Related Articles
November 7, 2018
Balloon pulmonary angioplasty for CTEPH
November 7, 2018
Endoscopic treatment of asthma
November 7, 2018
Gastroesophageal reflux, IPF and lessons learned
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

