Table: NCCN guidelines recommendations [9].
![ERS 2018 - oncology: Table 1. NCCN guidelines recommendations [9]](https://images.ctfassets.net/79wuvxegnvrv/lzy0NMis5kjOHaBrBf7Xo/e1dd5f036d50a922eee0f913b95249e8/Table_1._NCCN_guidelines_recommendations__9__.png)
Another subject Dr Mascaux discussed was the development of liquid biopsies, which determines the presence of circulating tumour cells in the blood [10]. This diagnostic tool is easy for both patient and doctor. In the AURA3 and FLAURA studies with osimertinib, tumour cells in the blood and in the tissue showed a high concordance with respect to their molecular biology. “However, a negative blood test could be false negative, so a tissue sample should still be analysed.”
Immunotherapy
Next to targeted therapies, another important development in the treatment of lung cancer, is that of immunotherapy. These drugs are directed either against PD-L1 which is expressed on T-cells, or PD-1 on tumour cells [11]. “In some patients, immunotherapy results in a durable response which can endure years after stopping the drug”, stated Dr Mascaux. “The more mutations are present in the tumour (i.e. higher mutational load), the better the response to immunotherapy. We need better biomarkers to predict the sensitivity for immunotherapy. Furthermore, combination therapy is needed to combat the heterogeneity of the tumour.”
- Solomon BJ, et al. N Engl J Med. 2014;371:2167-77.
- Shaw AT, et al. Lancet Oncol. 2017;18:874-886.
- Hida T, et al. Lancet. 2017;390:29-39.
- Peters S, et al. N Engl J Med. 2017;377:829-838.
- NCCN Clinical practice guidelines for NSCLC. 2017. www.nccn.org.
- Crowley E, et al. Nat Rev Clin Oncol. 2013;10:472-84.
- Herbst RS, et al. Nature. 2018;553:446-454.
Posted on
Previous Article
« Nintedanib and sildenafil Next Article
Endoscopic treatment of asthma »
« Nintedanib and sildenafil Next Article
Endoscopic treatment of asthma »
Related Articles
November 7, 2018
Macrolide antibiotics and trial with azithromycin
November 7, 2018
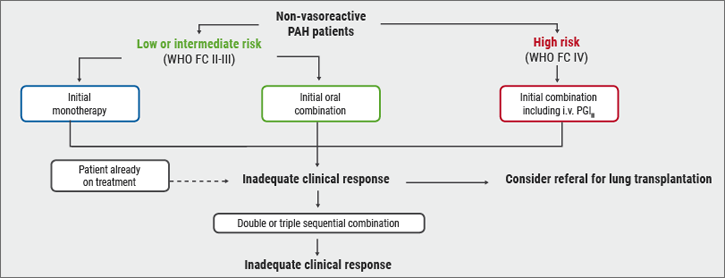
Risk stratification
November 7, 2018
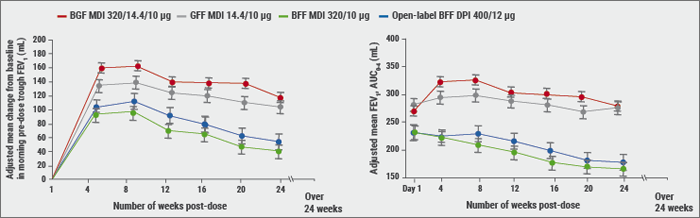
ICS: to use or not to use?
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

