Patients with advanced squamous cell carcinoma of the penis have a poor prognosis and high morbidity, due to progressive locoregional disease [1]. Previous experimental studies in this setting did not prove to be successful [2,3]. Pre-clinical studies show high rates of infiltrating immune cells and high PD-L1 expression, suggesting that immunotherapy may be beneficial for these patients [4].
The single-centre, phase 2 PERICLES trial (NCT03686332) assessed the activity of atezolizumab with or without radiotherapy to control locoregional lymph node disease in patients with advanced squamous cell carcinoma of the penis. The study enrolled 32 patients with stage IV disease. Any previous treatment except for immunotherapy was allowed. All participants were treated with atezolizumab for a maximum of 1 year. Participants (n=20) expected to benefit from radiotherapy for locoregional disease control (cohort A) received additional radiotherapy. The primary endpoint was 1-year PFS for the full cohort. The primary objective target was 1-year PFS ≥35%. Dr Hielke-Martijn de Vries (Netherlands Cancer Institute, the Netherlands) presented the results [5].
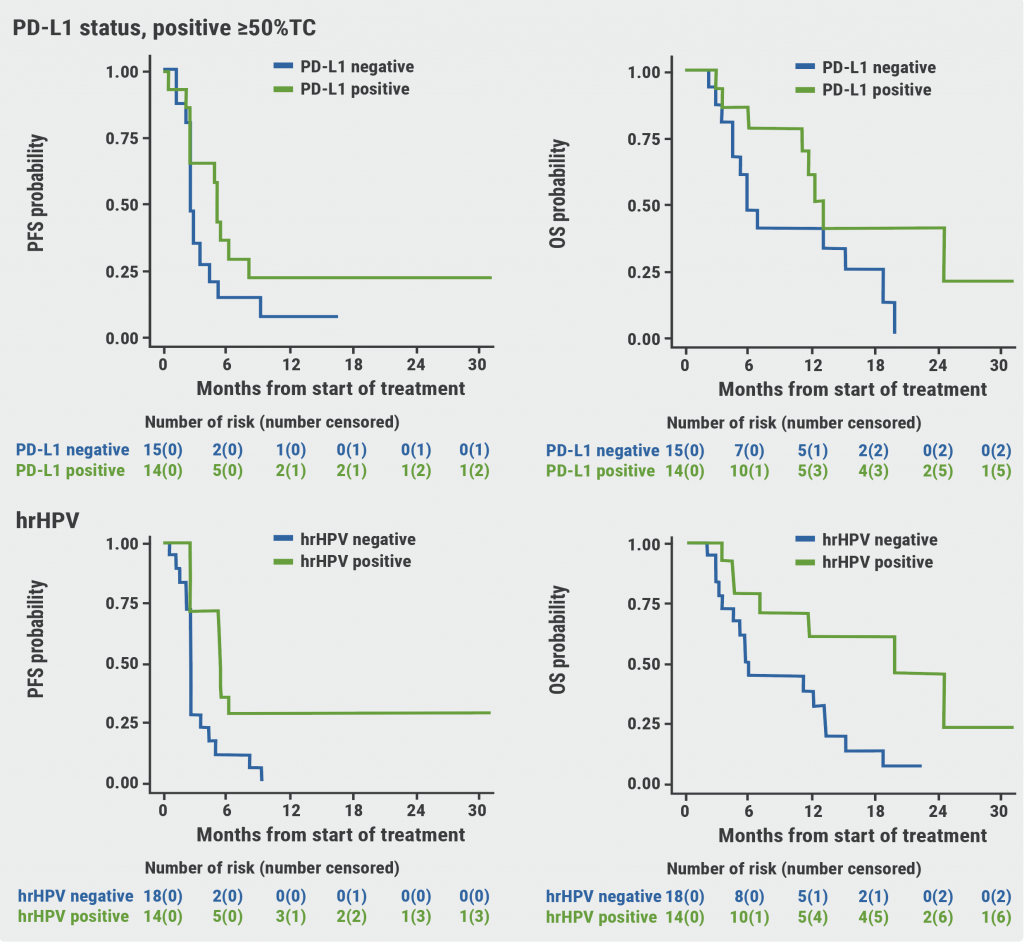
The median PFS was 2.5 months and 1-year PFS was 12.5%, which did not meet the primary objective. Participants with PD-L1 positivity and those who were positive for human papillomavirus (HPV) showed slightly higher PFS (see Figure). The 2 patients who responded well to treatment were both PD-L1-positive and high-risk HPV-positive. The response rate was 33% (23% partial response and 10% complete response). Median overall survival was 11.5 months.
Figure: Progression-free and overall survival of patients, stratified by PD-L1 and HPV status [5]
In terms of toxicity, 20 patients (63%) across both arms experienced any grade of immunotherapy-related adverse events while 3 (9.4%) experienced grade 3 or 4 adverse events. In the radiotherapy arm, 18 patients (90%) experienced radiotherapy-related adverse events, with 13 (65%) being grade 3 or 4.
“Despite some evidence of anti-tumour activity from atezolizumab, the trial did not meet its endpoint,” concluded Dr de Vries. “The combination with radiotherapy did not improve outcomes of immunotherapy.”
- Graafland NM, et al. Int J Cancer. 2011;128:426–432.
- Djajadiningrat RS, et al. Clin Genitour Cancer. 2015;13:44–49.
- Ottenhof S, et al. Eur Urol Suppl. 2019;18:e655.
- de Vries H-M, et al. Eur Urol Focus. 2019;5:718–721.
- de Vries H-M, et al. Clinical results of PERICLES: A phase II trial investigating atezolizumab +/- radiotherapy for advanced squamous cell carcinoma of the penis. Abstract 3, ASCO GU 2022, 17–19 February.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Biomarkers to distinguish necrosis from teratoma before pcRPLND in testicular cancer Next Article
HPV-positive and HPV-negative penile squamous cell carcinoma are molecularly distinct tumours »
« Biomarkers to distinguish necrosis from teratoma before pcRPLND in testicular cancer Next Article
HPV-positive and HPV-negative penile squamous cell carcinoma are molecularly distinct tumours »
Table of Contents: ASCO GU 2022
Featured articles
Prostate Cancer
First-line treatment with olaparib significantly improves PFS in mCRPC
First-line treatment with niraparib significantly improves PFS in HRR-mutated mCRPC
Darolutamide improves OS in mHSPC
Continued enzalutamide plus docetaxel offers clinical benefit for mCRPC patients who progress on enzalutamide
Radiohybrid PSMA PET imaging has favourable detection rate for prostate cancer recurrence
PSMA PET is a predictive biomarker in mCRPC progressing after docetaxel
Artificial intelligence improves prediction of long-term outcomes
Significant tumour response to neoadjuvant therapy in high-risk non-metastatic prostate cancer
Addition of abiraterone to ADT/docetaxel does not increase bone loss
Bavdegalutamide, a novel androgen receptor degrader, demonstrates clinical activity
Urothelial Carcinoma
No benefit of olaparib in previously untreated, platinum-ineligible, metastatic urothelial carcinoma
Rucaparib maintenance therapy extends PFS in platinum-responsive metastatic urothelial carcinoma
Positive efficacy and safety of N-803 plus BCG infusion in BCG-unresponsive NMIBC
Adding lenvatinib to pembrolizumab does not improve survival in advanced urothelial carcinoma
Maintenance niraparib fails to improve PFS in advanced urothelial cancer
First-line avelumab shows clinical activity in advanced urothelial carcinoma
Favourable pathologic response rate with neoadjuvant chemotherapy in high-risk upper tract urothelial carcinoma
Second-line nivolumab/ipilimumab boost improves ORR in metastatic urothelial carcinoma
Sacituzumab govitecan effective in platinum-refractory metastatic urothelial cancer
Neoadjuvant enfortumab vedotin promising in MIBC ineligible for cisplatin
Renal Cell Carcinoma
High-risk early RCC may benefit from neoadjuvant avelumab plus axitinib
DFS benefits with adjuvant pembrolizumab in RCC persist with longer follow-up
Biomarkers predict response to immune nivolumab (± ipilimumab) in advanced RCC
Combined nivolumab/axitinib treatment elicits good response in metastatic RCC
Folliculin mutations not associated with sporadic chromophobe RCC
Differential patterns of molecular alterations among sites of metastasis in RCC
Nivolumab monotherapy represents an alternative first-line treatment option for treatment-naïve mRCC
Penile & Testicular Cancer
HPV-positive and HPV-negative penile squamous cell carcinoma are molecularly distinct tumours
Atezolizumab does not improve survival in advanced penile cancer
Biomarkers to distinguish necrosis from teratoma before pcRPLND in testicular cancer
Related Articles
March 28, 2022
Rucaparib bests chemo in relapsed ovarian cancer

November 26, 2019
PFS benefit with niraparib as first-line maintenance in ovarian cancer
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

