To risk stratify patients, most guidelines, including the NCCN guidelines, use parameters like prostate-specific antigen (PSA), digital rectal exam (DRE), and Gleason score. However, this risk stratification tool is suboptimal in terms of performance. More recently, tissue assays have been developed assessing genomic biomarkers, but they are expensive and slow. AI tools leveraging digital pathology may provide solutions to these problems because they are quick, robust, and can be developed on thousands of patient samples without consuming any tissue. Dr Osama Mohamad (University of California San Francisco, CA, USA) presented a prognostic AI tool that was trained and validated using data from 5 phase 3 randomised trials, by leveraging multi-modal deep learning on digital histopathology [1].
Histopathology image data was generated from pre-treatment biopsy slides in 5 NRG Oncology radiotherapy prostate cancer trials (RTOG 9202, RTOG 9408, RTOG 9413, RTOG 9910, and RTOG 0126). Of 5,654 eligible patients, 16,204 digitalised histopathology slides of pre-treatment biopsy samples were randomly split into training (80%) and validation (20%) cohorts. A multi-modal artificial intelligence (MMAI) architecture was developed to take clinicopathologic and image-based data as input and predict binary outcomes.
After training, locking, and evaluating on the validation cohort, the MMAI prognostic model showed superior discrimination in comparison with the NCCN model (based on PSA, T-stage, and Gleason score) for 5- and 10-year distant metastases, 5- and 10-year biochemical failure, 10-year prostate cancer-specific survival, and 10-year overall survival (see Figure).
Figure: Evaluation of incremental benefit of various data [1]
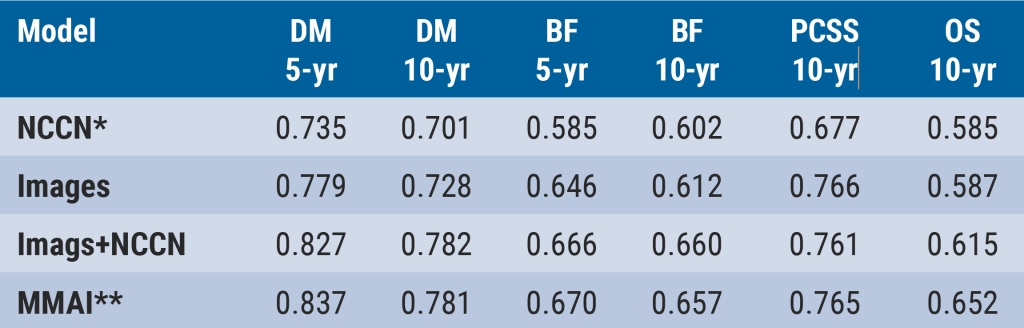
*NCCN = Gleason combined + baseline PSA + T-stage; **Pathology images + NCCN + Gleason primary + Gleason secondary + age; BF, biochemical failure; DM, distant metastases; OS, overall survival; PCSS, prostate cancer-specific survival
Based on these results, Dr Mohamad concluded that the AI tool can successfully predict long-term, clinically relevant outcomes for patients with prostate cancer.
- Esteva A, et al. Development and validation of a prognostic AI biomarker using multi-modal deep learning with digital histopathology in localized prostate cancer on NRG Oncology phase III clinical trials. Abstract 222, ASCO GU 2022, 17–19 February.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Significant tumour response to neoadjuvant therapy in high-risk non-metastatic prostate cancer Next Article
PSMA PET is a predictive biomarker in mCRPC progressing after docetaxel »
« Significant tumour response to neoadjuvant therapy in high-risk non-metastatic prostate cancer Next Article
PSMA PET is a predictive biomarker in mCRPC progressing after docetaxel »
Table of Contents: ASCO GU 2022
Featured articles
Prostate Cancer
First-line treatment with olaparib significantly improves PFS in mCRPC
First-line treatment with niraparib significantly improves PFS in HRR-mutated mCRPC
Darolutamide improves OS in mHSPC
Continued enzalutamide plus docetaxel offers clinical benefit for mCRPC patients who progress on enzalutamide
Radiohybrid PSMA PET imaging has favourable detection rate for prostate cancer recurrence
PSMA PET is a predictive biomarker in mCRPC progressing after docetaxel
Artificial intelligence improves prediction of long-term outcomes
Significant tumour response to neoadjuvant therapy in high-risk non-metastatic prostate cancer
Addition of abiraterone to ADT/docetaxel does not increase bone loss
Bavdegalutamide, a novel androgen receptor degrader, demonstrates clinical activity
Urothelial Carcinoma
No benefit of olaparib in previously untreated, platinum-ineligible, metastatic urothelial carcinoma
Rucaparib maintenance therapy extends PFS in platinum-responsive metastatic urothelial carcinoma
Positive efficacy and safety of N-803 plus BCG infusion in BCG-unresponsive NMIBC
Adding lenvatinib to pembrolizumab does not improve survival in advanced urothelial carcinoma
Maintenance niraparib fails to improve PFS in advanced urothelial cancer
First-line avelumab shows clinical activity in advanced urothelial carcinoma
Favourable pathologic response rate with neoadjuvant chemotherapy in high-risk upper tract urothelial carcinoma
Second-line nivolumab/ipilimumab boost improves ORR in metastatic urothelial carcinoma
Sacituzumab govitecan effective in platinum-refractory metastatic urothelial cancer
Neoadjuvant enfortumab vedotin promising in MIBC ineligible for cisplatin
Renal Cell Carcinoma
High-risk early RCC may benefit from neoadjuvant avelumab plus axitinib
DFS benefits with adjuvant pembrolizumab in RCC persist with longer follow-up
Biomarkers predict response to immune nivolumab (± ipilimumab) in advanced RCC
Combined nivolumab/axitinib treatment elicits good response in metastatic RCC
Folliculin mutations not associated with sporadic chromophobe RCC
Differential patterns of molecular alterations among sites of metastasis in RCC
Nivolumab monotherapy represents an alternative first-line treatment option for treatment-naïve mRCC
Penile & Testicular Cancer
HPV-positive and HPV-negative penile squamous cell carcinoma are molecularly distinct tumours
Atezolizumab does not improve survival in advanced penile cancer
Biomarkers to distinguish necrosis from teratoma before pcRPLND in testicular cancer
Related Articles
November 26, 2019
PFS extension with immunotherapy + chemotherapy in urothelial cancer
November 19, 2021
PARP inhibitor rechallenge improves PFS in ovarian cancer

March 14, 2022
ASCO GU 2022 Highlights Podcast
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

