The phase 3 MAIA (NCT02252172), ALCYONE (NCT02195479), and CASSIOPEIA (NCT02541383) studies established the superior PFS of daratumumab with standard-of-care versus standard-of-care alone for patients with newly diagnosed MM [1–3]. ALCYONE also established, for the first time, an OS benefit of a daratumumab-based regimen in newly diagnosed MM without eligibility for autologous stem cell transplantation [4]. In the previous MAIA update, OS data was not yet mature [5]. At the EHA 2021 congress, Prof. Thierry Facon (University of Lille, France) reported the updated efficacy and safety results from a pre-specified interim analysis after a median follow-up of 56.2 months [6].
MAIA is a multicentre, randomised, open-label, active-controlled phase 3 study of daratumumab plus lenalidomide and dexamethasone (D-Rd; n=364) versus lenalidomide and dexamethasone alone (Rd; n=365). Participants were randomised 1:1 and received treatment in cycles of 28 days until disease progression. Primary endpoint was PFS. Secondary endpoints included OS and overall response rate.
Median duration of follow-up was 56.2 months, with 42% of patients in the D-Rd arm and 18% of patients in the Rd arm remaining on treatment. Adverse events led to more patients discontinuing in the Rd-arm (23%) than in the D-Rd arm (13%). No new safety concerns were identified with longer follow-up.
The updated PFS data showed a 60-month PFS rate of 52.5% in D-Rd versus 28.7% in Rd (P<0.0001). Median PFS has not been reached with D-Rd so far and was 34.4 months with Rd. These results provide a new PFS benchmark in patients with newly diagnosed MM who are transplant ineligible. Overall response rate analysis showed that D-Rd induced deeper responses with significantly higher rates of complete response and very good partial response [2]. With >28 months of additional follow-up, responses deepened with continued daratumumab therapy.
D-Rd also demonstrated a significant benefit (P=0.0013) in OS, with a 32% reduction in the risk of death: 60-month OS rate was 66.3% in D-Rd and 53.1% in Rd (see Figure). Subgroup analysis confirmed OS benefit with D-Rd, as it was consistent across patient subgroups.
Figure: Overall survival data from an interim analysis show superior efficacy of D-Rd versus Rd [1]
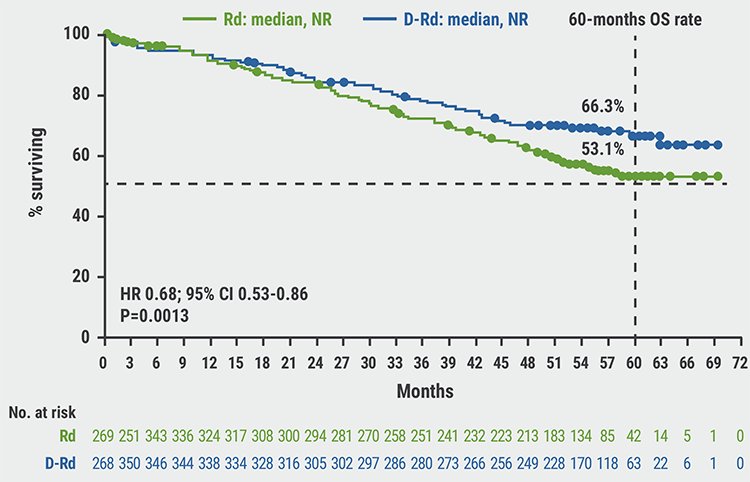 CI, confidence interval; D-Rd, daratumumab + lenalidomide and dexamethasone; HR, hazard ratio; NR, not reached; OS, overall survival; Rd, lenalidomide and dexamethasone.
CI, confidence interval; D-Rd, daratumumab + lenalidomide and dexamethasone; HR, hazard ratio; NR, not reached; OS, overall survival; Rd, lenalidomide and dexamethasone.
In summary, after almost 5 years of follow-up, a significant OS benefit of D-Rd versus Rd was demonstrated in patients with transplant-ineligible, newly diagnosed MM, representing a 32% reduction in the risk of death. The significant PFS benefit of D-Rd was maintained and no new safety concerns were identified with continuous therapy and longer follow-up. Prof. Facon concluded, “These results strongly support upfront D-Rd as new standard-of-care for patients with transplant-ineligible, newly diagnosed MM.”
- Mateos MV, et al. N Eng J Med 2018;378(6):518–28.
- Facon T, et al. N Eng J Med 2019;380(22):2104–15.
- Moreau P, et al. Lancet 2019;394(10192):29–38.
- Mateos MV, et al. Lancet 2020;395(10218):132–41.
- Kumar SK, et al. Abstract 2276, ASH 2020, 5–8 December.
- Facon T, et al. Overall survival results with daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: phase 3 MAIA study. P205-1, EHA 2021 Virtual Congress, 9–17 June.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« ANDROMEDA: Addition of daratumumab showed superior efficacy in patients with AL amyloidosis Next Article
Immune therapy of multiple myeloma »
« ANDROMEDA: Addition of daratumumab showed superior efficacy in patients with AL amyloidosis Next Article
Immune therapy of multiple myeloma »
Table of Contents: EHA 2021
Featured articles
Lymphoma
Immuno-oncology agents are effective in treating classic Hodgkin’s lymphoma
MATRix with ASCT: best long-term survival for primary CNS lymphoma
Naratuximab emtansine + rituximab safe and effective in diffuse large B-cell lymphoma
The journey ahead for CAR T-cell therapy in r/r follicular lymphoma
ZUMA-5 vs SCHOLAR-5: Axicabtagene ciloleucel significantly improves FL outcome
Promising chemo-free treatment options in r/r DLBCL
Leukaemia
Sabatolimab achieved durable responses in patients with high-risk MDS and AML
Final analysis of EURO-SKI: primary endpoints met in chronic myeloid leukaemia
Favourable outcomes with zanubrutinib versus ibrutinib in patients with r/r CLL
Oral azacitidine improves overall survival in patients with acute myeloid leukaemia
Reduced-intensity conditioning ASCT is effective in older patients with AML
ELEVATE-TN: Acalabrutinib shows long-term efficacy in chronic lymphocytic leukaemia
ELEVATE-RR: Acalabrutinib demonstrates similar efficacy and better safety versus ibrutinib
Fixed 12 cycles and MRD-guided venetoclax consolidation effective in CLL
GLOW: Ibrutinib + venetoclax showed superior PFS as first-line CLL treatment
Myeloma and Myelofibrosis
Novel targets in myelofibrosis: overview of emergent therapies
Immune therapy of multiple myeloma
MAIA results confirm superior efficacy of daratumumab with standard-of-care
ANDROMEDA: Addition of daratumumab showed superior efficacy in patients with AL amyloidosis
Thrombotic and Thrombocytopenic Disorders including COVID-19 related
Acquired TTP: new treatments and updated guidelines
Maternal screening to prevent foetal and neonatal alloimmune thrombocytopenia
Fostamatinib effectively increased platelet counts in immune thrombocytopenic purpura
Physiopathology of coagulopathy in haematological malignancies and COVID-19
Haemostatic abnormalities are associated with mortality in COVID-19
Mechanisms of COVID-19 vaccine-induced thrombotic thrombocytopenia
COVID-19 vaccine-induced immune thrombotic thrombocytopenia: discovery and diagnosis
Haemoglobinopathies
Luspatercept improved anaemia in patients with non-transfusion-dependent β-thalassaemia
Personalising treatment for sickle cell disease
Gene therapy: A promising approach for hereditary haemoglobinopathies
Related Articles
August 9, 2019
New sickle cell drug voxelotor boosts levels of haemoglobin
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

