Treatment cessation has become a realistic therapy goal since the introduction of TKIs for the treatment of CML, with treatment-free remission being achievable in up to 55% of patients with deep molecular response (DMR). However, little is known about prognostic indicators of sustained treatment-free remission. Thus, the multicentre, open-label, uncontrolled EURO-SKI study (NCT01596114), aimed to define prognostic markers besides other goals. Prof. Susanne Saußele (Heidelberg University, Germany) presented the final analysis after 3 years of follow-up [1].
EURO-SKI enrolled 728 adult CML patients on TKI treatment (≥3 years) and in confirmed DMR (≥1 year). The median age was 51 years, and 46.8% were women. First and secondary primary endpoints were molecular recurrence-free survival (MRecFS) at 6 and 36 months. Molecular recurrence was defined as BCR-ABL1 >0.1% (major molecular response [MMR] loss) at 1 time point. Patients were followed for 3 years after stop of TKI treatment. Median duration of TKI therapy was 7.5 years (3.1–22.6 years), with imatinib as first-line treatment in 93.7% of patients [2].
At 36 months, MRecFS was 48% (n=678; 95% CI 44–52) and molecular treatment-free survival (MRecTFS) was 46% (95% CI 43–50); 15 patients re-started TKI therapy without MMR loss. The cumulative incidence of MMR loss was 50% at 36 months (95% CI 46–54; see Figure). Of the 9 deaths, none were attributed to MMR relapse or CML.
Figure: Cumulative incidence of MMR loss after TKI treatment cessation [1]
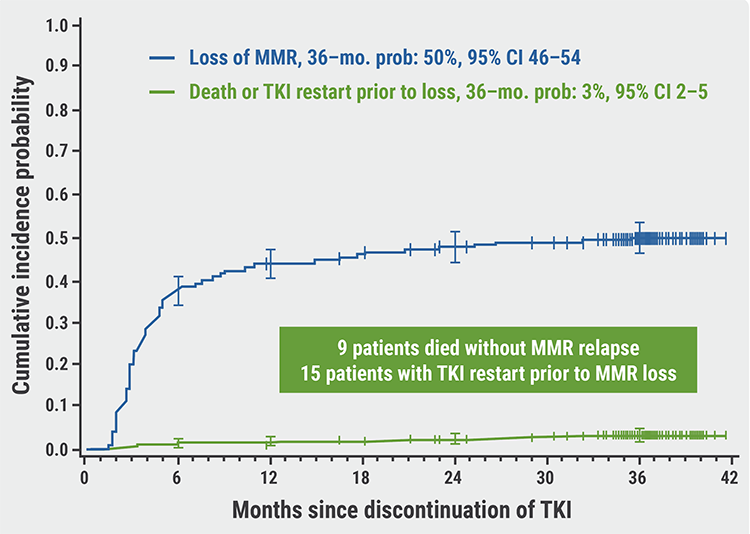 CI, confidence intervals; MMR, major molecular response; TKI, tyrosine kinase inhibitor.
CI, confidence intervals; MMR, major molecular response; TKI, tyrosine kinase inhibitor.
The first primary endpoint at 6-month was met: 434 out of 713 patients (61%; 95% CI 57–64) were in MMR or better, and the null hypothesis of ≤40% MMR maintenance at 6 months was rejected (P<0.0001). Similarly, the secondary primary endpoint of 36-month MRecFS was met: 309 out of 678 patients (46%; 95% CI 42–49) are still in MMR or better, and the null hypothesis of MMR maintenance at 36 months was rejected (P<0.0001). There were 17 patients who prematurely restarted TKI therapy and 33 patients with missing data.
Prof. Saußele also presented a preliminary analysis of prognostic factors. For MMR loss at 6 months, TKI treatment duration and DMR duration are the most important factors. For late MMR loss, TKI treatment duration before stop is the only relevant variable.
In summary, the primary endpoints of the EURO-SKI study were met and MRecFS probabilities were 62% and 46% after 6 and 36 months, respectively. First prognostic analyses support the importance of TKI treatment duration for early and late MMR loss, with further prognostic analyses to follow. The EURO-SKI study outlined important preconditions that can be employed as guidance for stopping criteria.
- Saußele S, et al. Final analysis of a pan European stop tyrosine kinase inhibitor trial in chronic myeloid leukemia: the EURO-SKI study. S152, EHA 2021 Virtual Congress, 9–17 June.
- Saußele S, et al. Lancet Oncol 2018;19(6):747-57.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Favourable outcomes with zanubrutinib versus ibrutinib in patients with r/r CLL Next Article
Sabatolimab achieved durable responses in patients with high-risk MDS and AML »
« Favourable outcomes with zanubrutinib versus ibrutinib in patients with r/r CLL Next Article
Sabatolimab achieved durable responses in patients with high-risk MDS and AML »
Table of Contents: EHA 2021
Featured articles
Lymphoma
Immuno-oncology agents are effective in treating classic Hodgkin’s lymphoma
MATRix with ASCT: best long-term survival for primary CNS lymphoma
Naratuximab emtansine + rituximab safe and effective in diffuse large B-cell lymphoma
The journey ahead for CAR T-cell therapy in r/r follicular lymphoma
ZUMA-5 vs SCHOLAR-5: Axicabtagene ciloleucel significantly improves FL outcome
Promising chemo-free treatment options in r/r DLBCL
Leukaemia
Sabatolimab achieved durable responses in patients with high-risk MDS and AML
Final analysis of EURO-SKI: primary endpoints met in chronic myeloid leukaemia
Favourable outcomes with zanubrutinib versus ibrutinib in patients with r/r CLL
Oral azacitidine improves overall survival in patients with acute myeloid leukaemia
Reduced-intensity conditioning ASCT is effective in older patients with AML
ELEVATE-TN: Acalabrutinib shows long-term efficacy in chronic lymphocytic leukaemia
ELEVATE-RR: Acalabrutinib demonstrates similar efficacy and better safety versus ibrutinib
Fixed 12 cycles and MRD-guided venetoclax consolidation effective in CLL
GLOW: Ibrutinib + venetoclax showed superior PFS as first-line CLL treatment
Myeloma and Myelofibrosis
Novel targets in myelofibrosis: overview of emergent therapies
Immune therapy of multiple myeloma
MAIA results confirm superior efficacy of daratumumab with standard-of-care
ANDROMEDA: Addition of daratumumab showed superior efficacy in patients with AL amyloidosis
Thrombotic and Thrombocytopenic Disorders including COVID-19 related
Acquired TTP: new treatments and updated guidelines
Maternal screening to prevent foetal and neonatal alloimmune thrombocytopenia
Fostamatinib effectively increased platelet counts in immune thrombocytopenic purpura
Physiopathology of coagulopathy in haematological malignancies and COVID-19
Haemostatic abnormalities are associated with mortality in COVID-19
Mechanisms of COVID-19 vaccine-induced thrombotic thrombocytopenia
COVID-19 vaccine-induced immune thrombotic thrombocytopenia: discovery and diagnosis
Haemoglobinopathies
Luspatercept improved anaemia in patients with non-transfusion-dependent β-thalassaemia
Personalising treatment for sickle cell disease
Gene therapy: A promising approach for hereditary haemoglobinopathies
Related Articles

July 1, 2021
EHA 2021 Highlights Podcast
August 5, 2021
Promising chemo-free treatment options in r/r DLBCL
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

