Dr Jeff Sharman (US Oncology Network, USA) discussed 4-year follow-up results of the multicentre, open-label, phase 3 ELEVATE-TN study (NCT02475681), which was designed to compare efficacy and safety of Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib ± obinutuzumab versus obinutuzumab + chlorambucil in patients with treatment-naïve CLL [1,2].
ELEVATE-TN enrolled 535 patients (median age 70 years) with previously untreated CLL with comorbidities and similar patient characteristics at baseline. Participants were randomised into 3 treatment arms: acalabrutinib + obinutuzumab (n=179), acalabrutinib monotherapy (n=179), and obinutuzumab + chlorambucil (n=177). Crossover from obinutuzumab + chlorambucil to acalabrutinib monotherapy was allowed after progression. The primary efficacy endpoint was progression-free survival (PFS) of acalabrutinib + obinutuzumab versus obinutuzumab + chlorambucil. Previously reported interim results demonstrated superior efficacy and acceptable tolerability of acalabrutinib ± obinutuzumab [2].
Median follow-up was 46.9 months (0.0–59.4 months). PFS was significantly prolonged in patients receiving acalabrutinib + obinutuzumab versus obinutuzumab + chlorambucil treatment (P<0.0001), as well as in acalabrutinib monotherapy versus obinutuzumab + chlorambucil (P<0.0001), and in acalabrutinib + obinutuzumab versus acalabrutinib monotherapy (P=0.0296). Median PFS was not reached in treatment arms acalabrutinib + obinutuzumab and acalabrutinib monotherapy, and median PFS was 27.8 months with obinutuzumab + chlorambucil. The 48-month PFS rate was 87% for acalabrutinib + obinutuzumab, 78% for acalabrutinib monotherapy and 25% for obinutuzumab + chlorambucil (see Figure). Efficacy of acalabrutinib + obinutuzumab was also superior in patients with 17p deletion and/or mutated TP53 or unmutated IGHV.
Figure: 4-year progression-free survival rates in ELEVATE-TN [1]
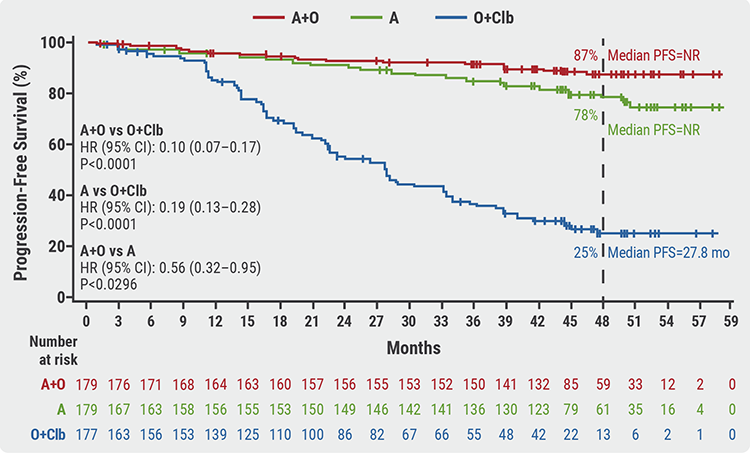 A, acalabrutinib; CI, confidence interval; Clb, chlorambucil; O, obinutuzumab; PFS, progression-free survival.
A, acalabrutinib; CI, confidence interval; Clb, chlorambucil; O, obinutuzumab; PFS, progression-free survival.The rate of complete response increased from the interim analysis to the 4-year follow-up analysis and was 30.7% for acalabrutinib + obinutuzumab, 11.2% for acalabrutinib monotherapy, and 13.0% for obinutuzumab + chlorambucil. The minimal residual disease status in patients also showed favourable outcomes for acalabrutinib + obinutuzumab. Median overall survival was not reached in any treatment arm. At 48 months, overall survival rates were 93%, 88%, and 88%, which was not statistically significantly different. At 60 months, there were still notably fewer deaths with acalabrutinib + obinutuzumab compared with other treatment arms.
More than 25% of patients in any treatment group experienced adverse events, as previously reported [2]. Neutropenia was more frequently observed in patients treated with acalabrutinib + obinutuzumab versus acalabrutinib monotherapy-treated patients, but less frequently than with obinutuzumab + chlorambucil. Most of the adverse events occurred in the first year of treatment and declined over time. No new safety signals were observed.
Dr Sharman concluded, “acalabrutinib with or without obinutuzumab demonstrates durable disease control, tolerability, and flexibility to tailor treatment as a monotherapy or combination therapy in treatment-naïve CLL patients.”
- Sharman JP, et al. Acalabrutinib ± obinutuzumab vs obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: ELEVATE-TN 4-year follow-up. P409-4, EHA 2021 Virtual Congress, 9–17 June.
- Sharman JP, et al. Lancet 2020;395:1278–91.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« ELEVATE-RR: Acalabrutinib demonstrates similar efficacy and better safety versus ibrutinib Next Article
Reduced-intensity conditioning ASCT is effective in older patients with AML »
« ELEVATE-RR: Acalabrutinib demonstrates similar efficacy and better safety versus ibrutinib Next Article
Reduced-intensity conditioning ASCT is effective in older patients with AML »
Related Articles
June 15, 2023
Next-generation cell therapies for cancer: CAR-NK cells
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

