https://doi.org/10.55788/d673eaa0
Both the JAK3/TEC and the JAK1/TYK2 pathways are implicated in the pathogenesis of UC through regulation of pro-inflammatory cytokine signalling and CD8+ T-cell cytotoxicity. This was the rationale to assess the efficacy and safety of the JAK3/TEC inhibitor ritlecitinib and the JAK1/TYK2 inhibitor brepocitinib in patients with UC in the phase 2b VIBRATO study (NCT02958865). In this trial, both small molecules were more effective during the 8-week induction period compared with placebo, with an acceptable safety profile. Dr Kenneth Hung (Pfizer, MA, USA) pointed out that, in the induction phase, modified remission at week 8 was high, as 38% and 25.5% of patients achieved endoscopic improvement with ritlecitinib and brepocitinib respectively [1]. “Next we wanted to evaluate efficacy and safety in a 24-week period,” he explained.
In the 8-week induction phase, both agents were given in 3 doses (20, 70, or 200 mg ritlecitinib once daily or 10, 30, or 60 mg brepocitinib once daily) and compared with placebo once daily. In the chronic dosing therapy phase, all participants continued in their respective treatment cohorts to receive oral ritlecitinib 50 mg or brepocitinib 30 mg once daily for 24 weeks. At week 32, efficacy was assessed with the total Mayo score (primary endpoint), clinical remission, modified remission, and endoscopic improvement. A total of 279 participants received treatment in the chronic period. Baseline characteristics were generally similar across treatment groups. About 40% to 65% of patients had already received biologics.
“We saw great improvement from week 8 to 32 for those with lower induction dosing. However, for those cohorts with higher induction doses, we saw that the improvement was less dramatic from week 8 to week 32,” Dr Hung explained the changes in the total Mayo score (see Figure).
Figure: Improvements in total Mayo Score after 32 weeks are more pronounced in patients treated with low induction doses [1]
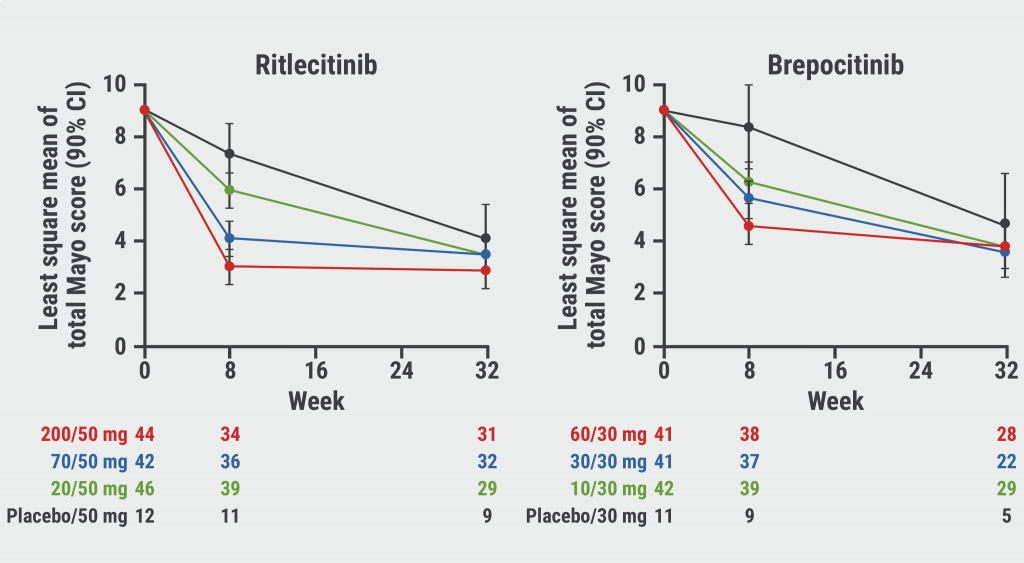
Similar patterns were seen for clinical remission (defined as total Mayo Score ≤2; no individual subscore >1; rectal bleeding subscore=0) and modified remission (modified Mayo Score [total Mayo Score without Physician Global Assessment]: stool frequency subscore ≤1; rectal bleeding subscore=0; endoscopic subscore ≤1). Rates of clinical remission and modified clinical remission generally continued to improve in groups receiving a lower induction dose. “These improvements were somewhat blunted in the higher induction dose groups,” Dr Hung explained.
Therapy with both agents also led to endoscopic improvements (Mayo endoscopic subscore ≤1). Changes generally continued to improve from week 8 to 32 in groups receiving a lower induction dose. Again, this improvement was generally more modest from week 8 through 32 in higher induction dose groups.
Dr Hung pointed out that both drugs were relatively safe and well tolerated across the groups. The most commonly reported adverse events included chronic urticaria, nasopharyngitis, arthralgia, and pyrexia. Moreover, there were no clinically significant observations for laboratory parameters. No thromboembolic events or malignancies were reported during the chronic period. However, there was a herpes signal: 2 participants in the chronic period suffered from herpes simplex (1 in each arm), another 4 participants had herpes zoster in the chronic dosing period (3 in the ritlecitinib and 1 in the brepocitinib arm). Only 1 of these events was moderate, all others were mild and did not lead to treatment discontinuation.
These positive study results support the further development of ritlecitinib and brepocitinib in patients with moderate-to-severe UC.
- Sandborn WJ, et al. Ritlecitinib and brepocitinib in patients with moderately to severely active Ulcerative Colitis: chronic therapy data from the VIBRATO umbrella study. OP090, UEG Week 2022, 8–11 October, Vienna, Austria.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Another chance for TYK2 inhibition in UC Next Article
Sustained maintenance results with upadacitinib in UC »
« Another chance for TYK2 inhibition in UC Next Article
Sustained maintenance results with upadacitinib in UC »
Table of Contents: UEGW 2022
Featured articles
IBD in 2022
Fast recapture of response with ozanimod after withdrawal in UC
Ozanimod treatment prompted substantial response after failure of response to induction
Etrasimod shows advantage over placebo in UC
Etrasimod reduces adaptive immune cells in the periphery in UC
Favourable maintenance rates for risankizumab also in delayed responders with CD
IL-23 inhibition reduces inflammatory biomarkers in pre-treated UC
Maintained symptom control with mirikizumab in UC
Mirikizumab successfully resolves active histologic inflammation in UC
Upadacitinib for CD: remarkable efficacy in induction therapy
Sustained maintenance results with upadacitinib in UC
Another chance for TYK2 inhibition in UC
Small molecule obefazimod shows promise in UC
Pivotal results of etrolizumab for CD partly disappointing
Better results for vedolizumab in early CD
Some patients with limited CD may benefit from an early surgical intervention
Dose-interval of adalimumab might be prolonged in CD patients in stable remission
What Is Hot in Upper GI Disorders?
Less ulcer bleeds early after H. pylori eradication in aspirin users
Dupilumab effective in paediatric patients with eosinophilic oesophagitis
Neoplasia in Barrett’s oesophagus: the earlier the intervention, the better the long-term outcome
Hepatology in 2022
Favourable pancreatitis outcomes with procalcitonin-based algorithm to guide antibiotic use
Portal hypertension is associated with poor prognosis in cirrhotic patients
Chances of transplant-free survival in PSC enhanced by colectomy with ileostomy
SARS-CoV-2: Booster doses of key importance for cirrhotic patients
What Is New in Pancreatic Cancer and Pancreatitis?
Fewer long-term interventions after delayed drainage in necrotising pancreatitis
Detection of Europe´s deadliest cancer: much room for improvement
Colorectal Carcinoma: Improving Diagnosis and Therapy
Immunotherapy response may be modulated by microbiome
Computer-aided colonoscopies improved adenoma detection rates
Screening-detected colorectal cancers may have superior surgical outcomes
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

