https://doi.org/10.55788/6205580f
In the phase 3 ADVANCE (NCT03105128) and MOTIVATE (NCT03104413) trials, patients with CD who did not respond to a first induction of 12 weeks with the IL-23 inhibitor risankizumab, received a second 12-week period of induction treatment. Those who achieved a ≥30% decrease of average daily stool frequency (SF) and/or a ≥30% reduction in their average daily abdominal pain score (APS) were called ‘delayed responders’ and were eligible to enter the FORTIFY trial (NCT03105102). These patients continued to receive either 180 or 360 mg of intravenous risankizumab. Prof. Geert D’Haens (Amsterdam UMC, the Netherlands) shared results on the long-term efficacy and safety of risankizumab at week 52 of maintenance treatment in those ‘delayed responders’ [1].
The analysis included 63 participants (30 on 180 mg, 33 on 360 mg) with an average age between 38.5–40.4 years, and 36.4–40.0% were women. Most participants had a history of failure to anti-TNF treatment (Anti-TNF History failure of 1: 72.2% [180 mg group] and 62.5% [360 mg group]; Anti-TNF History failure of >1: 27.8% [180 mg group] and 37.5% [360 mg group], but also previous therapy with vedolizumab (failure 38.9% and 25% for low and high dose group) or ustekinumab (failure 16.7% and 12.5% for low and high dose group) was reported. Mean Crohn’s Disease Activity Index (CDAI) was 288.7 and 291.1 for the low- and high-dose group and mean Simple Endoscopic Score for Crohn’s Disease (SES-CD) was 11.6 and 13.6, respectively.
At week 52, a CDAI response (CDAI ≥100 points from baseline of the induction study) was present in 53.3% (180 mg) and 75.8% (360 mg) of the participants, while 56.7% and 66.7% achieved CDAI remission (CDAI <150). Clinical remission rates in the SF/APS outcome (average daily SF ≤2.8 and not worse than baseline and average daily AP score ≤1 and not worse than baseline) were 43.3% on the lower and 54.5% on the higher dose (see Figure). “Of course, the study was not powered to show a dose-response curve, but there is a numerical superiority for the higher dose of risankizumab. Remember that these are kind of refractory patients, they needed 2 induction treatments before they had a significant drop in their CDAI,” Prof. D’Haens commented. In the composite endpoint of deep remission (CDAI clinical remission plus endoscopic remission), rates of 40.0 and 39.4% were observed for the 180 mg and 360 mg regimens. The respective results for endoscopic response and endoscopic remission in the 360 versus the 180 mg arms were 45.5 versus 36.7% and 42.4 versus 40.0%. An ulcer-free endoscopy was attained by 24.2% (360 mg) and 27.6% (180 mg). The safety summary did not reveal new safety risks and risankizumab was overall well tolerated.
Figure: Clinical endpoints at week 52 in risankizumab delayed responders [1]
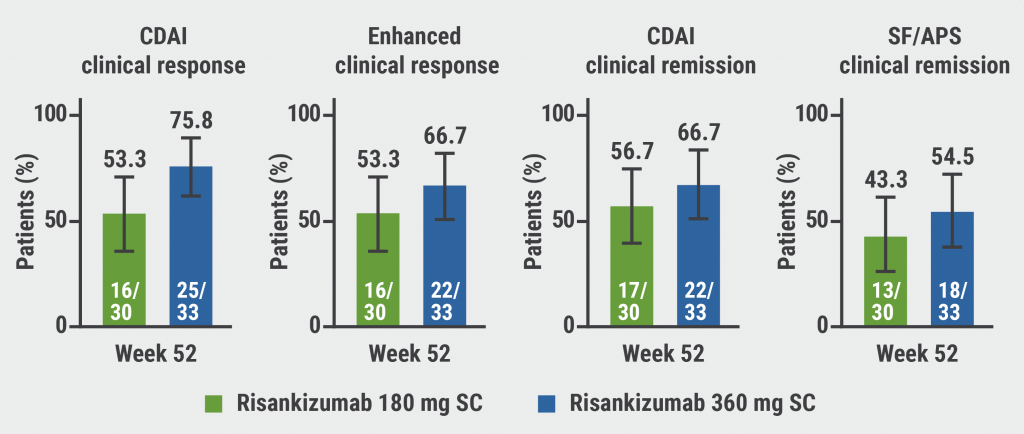
CDAI, Crohn’s Disease Activity Index; SF, stool frequency; APS, abdominal pain score; SC, subcutaneous.
“These findings underscore an additional clinical benefit of initiating maintenance dosing of subcutaneous risankizumab, following intravenous induction, even in the patients who do not respond after 12 weeks,” Prof. D’Haens concluded.
- D’Haens G, et al. 52-weeks risankizumab subcutaneous maintenance dosing is efficacious and well tolerated in patients with moderate-to-severe Crohn’s disease who had delayed response to 12 weeks IV risankizumab induction. OP126, UEG Week 2022, 8–11 October, Vienna, Austria.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« IL-23 inhibition reduces inflammatory biomarkers in pre-treated UC Next Article
Etrasimod reduces adaptive immune cells in the periphery in UC »
« IL-23 inhibition reduces inflammatory biomarkers in pre-treated UC Next Article
Etrasimod reduces adaptive immune cells in the periphery in UC »
Table of Contents: UEGW 2022
Featured articles
IBD in 2022
Fast recapture of response with ozanimod after withdrawal in UC
Ozanimod treatment prompted substantial response after failure of response to induction
Etrasimod shows advantage over placebo in UC
Etrasimod reduces adaptive immune cells in the periphery in UC
Favourable maintenance rates for risankizumab also in delayed responders with CD
IL-23 inhibition reduces inflammatory biomarkers in pre-treated UC
Maintained symptom control with mirikizumab in UC
Mirikizumab successfully resolves active histologic inflammation in UC
Upadacitinib for CD: remarkable efficacy in induction therapy
Sustained maintenance results with upadacitinib in UC
Another chance for TYK2 inhibition in UC
Small molecule obefazimod shows promise in UC
Pivotal results of etrolizumab for CD partly disappointing
Better results for vedolizumab in early CD
Some patients with limited CD may benefit from an early surgical intervention
Dose-interval of adalimumab might be prolonged in CD patients in stable remission
What Is Hot in Upper GI Disorders?
Less ulcer bleeds early after H. pylori eradication in aspirin users
Dupilumab effective in paediatric patients with eosinophilic oesophagitis
Neoplasia in Barrett’s oesophagus: the earlier the intervention, the better the long-term outcome
Hepatology in 2022
Favourable pancreatitis outcomes with procalcitonin-based algorithm to guide antibiotic use
Portal hypertension is associated with poor prognosis in cirrhotic patients
Chances of transplant-free survival in PSC enhanced by colectomy with ileostomy
SARS-CoV-2: Booster doses of key importance for cirrhotic patients
What Is New in Pancreatic Cancer and Pancreatitis?
Fewer long-term interventions after delayed drainage in necrotising pancreatitis
Detection of Europe´s deadliest cancer: much room for improvement
Colorectal Carcinoma: Improving Diagnosis and Therapy
Immunotherapy response may be modulated by microbiome
Computer-aided colonoscopies improved adenoma detection rates
Screening-detected colorectal cancers may have superior surgical outcomes
Related Articles
January 24, 2022
Yes signaling pathway implicated in liver cancer development
November 26, 2019
Nivolumab improves OS in advanced oesophageal cancer

December 7, 2023
How to deal with at-risk patients above the CRC screening age limit?
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

