https://doi.org/10.55788/45197538
Immune checkpoint inhibitors (ICIs) were a huge step forward in oncology. They augment T cell-mediated immune responses to tumours, resulting in improved overall survival of cancer patients at advanced stages in certain types of colorectal cancer. However, durable responses are only achieved by a subset of patients. Recent research has shown that the intestinal microbiome might modulate responses to ICIs [1]. Thus, Dr Johannes Björk (University Medical Center Groningen, the Netherlands) set out to analyse longitudinal changes in the gut microbiome in response to ICB treatment, drawing on data from the observational PRIMM study (NCT03643289).
The researchers profiled the gut microbiome of advanced melanoma patients (n=175) undergoing ICB at cancer centres in the UK and the Netherlands. Shotgun metagenomic sequencing was performed on stool samples to compare the microbiome before and during treatment up to 4 times and to explore the correlation with treatment success, measured in terms of 12-month progression-free survival. The researchers used a regression model with higher-order interactions to estimate how bacterial species and metabolic pathways changed in abundance in different (non-)responder strata from baseline to after the final treatment injection. They also disentangled the longitudinal effect of the following treatment characteristics: the use of single (PD-1) or combination (PD-1 and CTLA-4) ICB, prior exposure to antibiotics or proton pump inhibitors (PPIs), and the effect of immune-related adverse events such as colitis.
The results confirmed that some of the previously reported microbial biomarkers at baseline, such as Faecalibacterium prausnitzii and Bifidobacterium longum, also increased longitudinally during treatment. However, these associations were often influenced by concomitant treatment characteristics. For example, microbiota differed between single ICB and combination ICB in patients that took no PPIs and no antibiotics. Compared with non-responders, a higher and increasing abundance of butyrate producers from the Lachnospiraceae family was identified in responders on single ICB over the entire treatment period. “Of course, this is information that we couldn’t have known from previous baseline predictions alone,” Dr Björk pointed out. In contrast, non-response was associated with a higher and increasing abundance of several Bacteroides species, some of which have already been identified in non-responders in previous baseline studies.
Dr Björk also emphasised the effect of confounders like PPI or antibiotic use (see Figure). PPI use was associated with a longitudinal increase in Klebsiella pneumoniae in non-responders that was not seen in non-responders not taking PPIs. Finally, the researchers compared longitudinal microbiota changes in responders who developed colitis compared with those who did not. In general, immunotherapy-induced colitis resembles the gut microbiome in IBD. Bacteriodes caccae was enriched in responders that developed colitis compared with those who did not. Presence of Faecalibacterium prausnitzii, also a butyrate producer, was high and stable in responders resistant to colitis compared with those developing colitis.
Figure: Confounders and outcomes during immunotherapy [2]
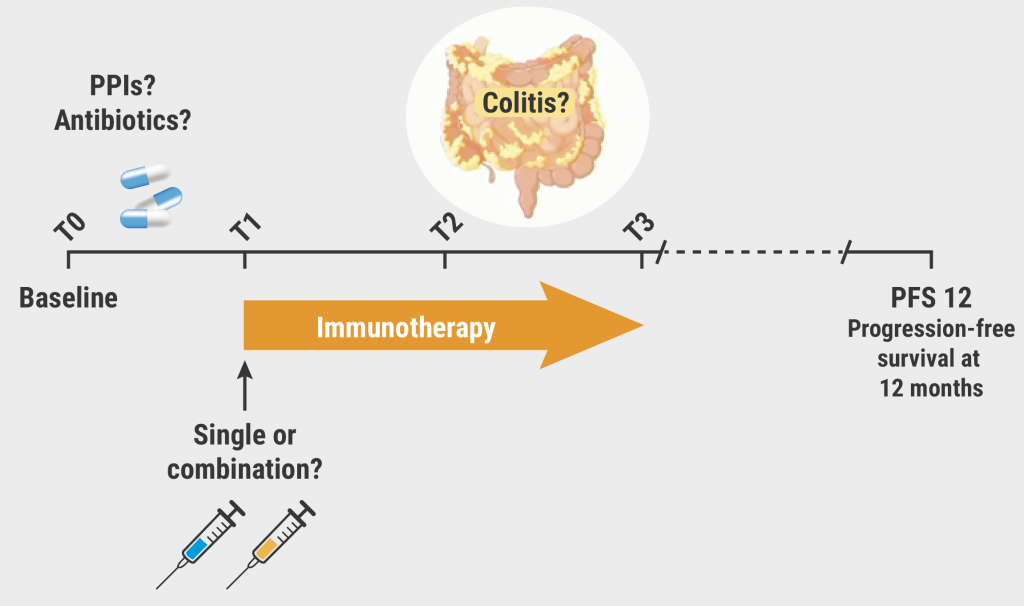
PPIs, proton pump inhibitors.
“Microbiome-based interventions should not solely rely on baseline biomarkers. This is key to increasing the efficacy of ICIs. We have to understand the dynamic of the system we want to modulate,” Dr Björk concluded.
- Hayase E, Jenq RR. Genome Med 2021;13:107.
- Björk J, et al. Longitudinal changes in the gut microbiome in response to immune checkpoint blockade. OP042, UEG Week 2022, 8–11 October, Vienna, Austria.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Computer-aided colonoscopies improved adenoma detection rates Next Article
Letter from the Editor »
« Computer-aided colonoscopies improved adenoma detection rates Next Article
Letter from the Editor »
Table of Contents: UEGW 2022
Featured articles
IBD in 2022
Fast recapture of response with ozanimod after withdrawal in UC
Ozanimod treatment prompted substantial response after failure of response to induction
Etrasimod shows advantage over placebo in UC
Etrasimod reduces adaptive immune cells in the periphery in UC
Favourable maintenance rates for risankizumab also in delayed responders with CD
IL-23 inhibition reduces inflammatory biomarkers in pre-treated UC
Maintained symptom control with mirikizumab in UC
Mirikizumab successfully resolves active histologic inflammation in UC
Upadacitinib for CD: remarkable efficacy in induction therapy
Sustained maintenance results with upadacitinib in UC
Another chance for TYK2 inhibition in UC
Small molecule obefazimod shows promise in UC
Pivotal results of etrolizumab for CD partly disappointing
Better results for vedolizumab in early CD
Some patients with limited CD may benefit from an early surgical intervention
Dose-interval of adalimumab might be prolonged in CD patients in stable remission
What Is Hot in Upper GI Disorders?
Less ulcer bleeds early after H. pylori eradication in aspirin users
Dupilumab effective in paediatric patients with eosinophilic oesophagitis
Neoplasia in Barrett’s oesophagus: the earlier the intervention, the better the long-term outcome
Hepatology in 2022
Favourable pancreatitis outcomes with procalcitonin-based algorithm to guide antibiotic use
Portal hypertension is associated with poor prognosis in cirrhotic patients
Chances of transplant-free survival in PSC enhanced by colectomy with ileostomy
SARS-CoV-2: Booster doses of key importance for cirrhotic patients
What Is New in Pancreatic Cancer and Pancreatitis?
Fewer long-term interventions after delayed drainage in necrotising pancreatitis
Detection of Europe´s deadliest cancer: much room for improvement
Colorectal Carcinoma: Improving Diagnosis and Therapy
Immunotherapy response may be modulated by microbiome
Computer-aided colonoscopies improved adenoma detection rates
Screening-detected colorectal cancers may have superior surgical outcomes
Related Articles

October 13, 2022
UEGW 2022 Highlights Podcast
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

