https://doi.org/10.55788/6f4de4da
According to a retrospective study, dose de-escalation of adalimumab is possible in patients with CD [1]. One possible way of achieving this is simply increasing the dose interval. Possible advantages of this approach are decreasing adverse events linked to biological therapy. Cost-savings are another important advantage of a longer dose interval: “There are not only side effects for the patient, but also for society, because we know that with an increasing uptake of biologics, healthcare costs are rising rapidly,” Dr Reinier van Linschoten (Franciscus Gasthuis en Vlietland, the Netherlands) explained. An open-label, multicentre, randomised-controlled, non-inferiority LADI trial (NCT03172377) assessed the clinical outcomes of an increased adalimumab dose interval compared with conventional dosing in CD patients in stable remission [2]. All 174 participants were in steroid-free clinical remission while on adalimumab maintenance therapy (40 mg adalimumab, every other week, for at least 9 months). Participants were randomised to increase adalimumab dose interval from 2 to 3 and then to 4 weeks (n=113), or to continue the conventional dose interval of 2 weeks (n=61). The primary study endpoint was the incidence of persistent flares.
“A dose extension was possible in most of the patients in the intervention group, only 10% had to go back to the conventional dose interval,” Dr van Linschoten explained. Four patients in the intervention group and 1 patient in the control group were excluded from the analysis for not meeting the inclusion criteria. The cumulative incidence of persistent flares at week 48 in the intervention group (3/109) was non-inferior compared with the control group (0/60). In addition, the cumulative incidence of transient flares was similar between the groups (pooled adjusted risk difference [paRD] 2.68%; 95% CI -0.93–6.30; see Figure). At week 48, 92% of the control group and 72% of the intervention group were in clinical remission (paRD -16.3%; 95% CI -30.9 to -1.82). Neither disease activity nor quality-of-life significantly differed between the control and the intervention group. However, the intervention group used significantly more rescue mediation. Participants in the intervention group showed an increase in GI disorders, mainly mild GI side effects. A difference was also noted regarding the infection-associated adverse events: Per 100 person-years, 60 infection-related adverse events occurred in the intervention group versus 75 in the control group.
Figure: Disadvantages of a longer dose interval are an increase in escape medication and fewer patients in clinical remission [2]
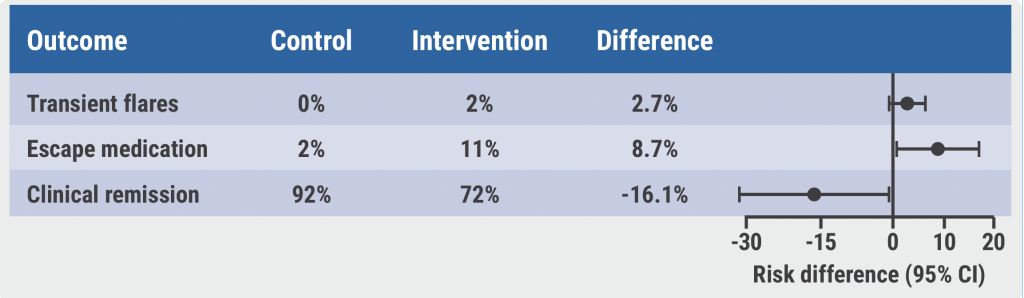
“Increasing the adalimumab dose interval is a possible treatment strategy. However, there are some negative consequences like an increase in escape medication and fewer patients in clinical remission at week 48,” Dr van Linschoten concluded. Therefore, this approach should be discussed individually with the patient.
- Van Steenbergen S, et al. Aliment Pharmacol Ther. 2017;45:923–932.
- Van Linschoten RCA, et al. Clinical outcomes of increased versus conventional adalimumab dose inervals in patients with Crohn´s disease in stable remission. OP106, UEG Week 2022, 8–11 October, Vienna, Austria.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Glucocorticoids in rheumatic diseases: Is there a skeletal sparing dose? Next Article
Some patients with limited CD may benefit from an early surgical intervention »
« Glucocorticoids in rheumatic diseases: Is there a skeletal sparing dose? Next Article
Some patients with limited CD may benefit from an early surgical intervention »
Table of Contents: UEGW 2022
Featured articles
IBD in 2022
Fast recapture of response with ozanimod after withdrawal in UC
Ozanimod treatment prompted substantial response after failure of response to induction
Etrasimod shows advantage over placebo in UC
Etrasimod reduces adaptive immune cells in the periphery in UC
Favourable maintenance rates for risankizumab also in delayed responders with CD
IL-23 inhibition reduces inflammatory biomarkers in pre-treated UC
Maintained symptom control with mirikizumab in UC
Mirikizumab successfully resolves active histologic inflammation in UC
Upadacitinib for CD: remarkable efficacy in induction therapy
Sustained maintenance results with upadacitinib in UC
Another chance for TYK2 inhibition in UC
Small molecule obefazimod shows promise in UC
Pivotal results of etrolizumab for CD partly disappointing
Better results for vedolizumab in early CD
Some patients with limited CD may benefit from an early surgical intervention
Dose-interval of adalimumab might be prolonged in CD patients in stable remission
What Is Hot in Upper GI Disorders?
Less ulcer bleeds early after H. pylori eradication in aspirin users
Dupilumab effective in paediatric patients with eosinophilic oesophagitis
Neoplasia in Barrett’s oesophagus: the earlier the intervention, the better the long-term outcome
Hepatology in 2022
Favourable pancreatitis outcomes with procalcitonin-based algorithm to guide antibiotic use
Portal hypertension is associated with poor prognosis in cirrhotic patients
Chances of transplant-free survival in PSC enhanced by colectomy with ileostomy
SARS-CoV-2: Booster doses of key importance for cirrhotic patients
What Is New in Pancreatic Cancer and Pancreatitis?
Fewer long-term interventions after delayed drainage in necrotising pancreatitis
Detection of Europe´s deadliest cancer: much room for improvement
Colorectal Carcinoma: Improving Diagnosis and Therapy
Immunotherapy response may be modulated by microbiome
Computer-aided colonoscopies improved adenoma detection rates
Screening-detected colorectal cancers may have superior surgical outcomes
Related Articles
October 23, 2019
IBD prevalence 3 times higher than estimated and expected to rise
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

