Prof. Paul Armstrong (University of Alberta, Canada) presented the results of the 42-country Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA) trial, which was designed to evaluate vericiguat compared with placebo among patients with chronic heart failure due to reduced ejection fraction (HFrEF). Vericiguat is a first-in-class stimulator of soluble guanylate cyclase (sGC) activity that also enhances sGC sensitivity to endogenous nitric oxide (NO). Deficiency in sGC-derived cyclic guanosine monophosphate (cGMP) causes both myocardial dysfunction and impaired endothelium-dependent vasomotor regulation. Hence, restoration of sufficient NO-sGC–cGMP signalling has been proposed as an important treatment target in HF.
The primary composite outcome of this trial was cardiovascular death or first HF hospitalisation. The phase 3, randomised, double-blind, placebo-controlled trial included 5,050 patients with chronic HF (defined as New York Heart Association [NYHA] functional class II-IV, left ventricular ejection fraction <45%, and guideline-based HF therapies), after a worsening event (defined as an HF hospitalisation or receiving an intravenous diuretic for HF without hospitalisation) with very elevated natriuretic peptides (i.e. brain natriuretic peptide [BNP] or N-terminal [NT]-proBNP). They were randomly assigned to receive either vericiguat (n=2,526) or placebo (n=2,524) + guideline-based HF therapy. Patients received vericiguat initially at 2.5 mg/day, titrated over a month to a target dose of 10 mg/day. Baseline characteristics were evenly balanced between the 2 groups. Median follow-up was 10.8 months.
The primary composite endpoint was reached by 35.5% of vericiguat patients and 38.5% of placebo patients (HR 0.90; 95% CI 0.82-0.98; P=0.019), a 10% lower risk of cardiovascular death or HF hospitalisation (see Figure). However, the individual component analysis showed that these data were primarily driven by the reduction of total hospitalisations for heart among patients taking vericiguat (HR 0.91; 95% CI 0.84-0.99; P=0.02). These data indicate an absolute risk reduction of 4.2 per 100 patient-years in the vericiguat arm. Roughly 24 patients with chronic HFrEF would need to be treated to prevent 1 cardiovascular death/HF hospitalisation.
Figure: Primary composite endpoint: Cardiovascular death or first heart failure hospitalisation [1]
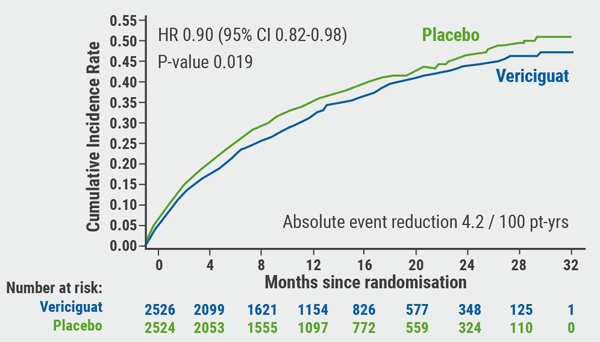
No significant difference in cardiovascular death was observed: 12.9% for the vericiguat arm versus 13.9% for the placebo arm (HR 0.93; 95% CI 0.81-1.06; P=0.269). Regarding safety, vericiguat titrated to 10 mg was generally safe and well-tolerated, although reports of hypotension and syncope were numerically higher with vericiguat.
In conclusion, vericiguat may represent an expansion of options for patients with a recent worsening HF event, especially because it is a once-daily oral medicine, easy to titrate, generally safe and well tolerated, and does not require monitoring of renal function or electrolytes. These data are particularly relevant because hospitalisation for worsening HF represents a “major inflection point” in the natural history of the condition, which is characterised by a marked change in the risk of future hospitalisations and mortality. To date, no prior therapies have attenuated this risk, but vericiguat might be the first to alter the natural history of HF after a patient has had a worsening event. Furthermore, this trial represents the feasibility of modulating the bioavailability of NO as a novel therapeutic approach for HF, as well as potentially other cardiovascular conditions characterised by insufficient NO-sGC–cGMP signalling.
- Armstrong PW, et al. Abstract 402-08. ACC/WCC 28-30 March 2020.
Posted on
Previous Article
« No role for sodium nitrite in out-of-hospital cardiac arrest Next Article
Sacubitril-valsartan in hospitalized HF patients could have benefits »
« No role for sodium nitrite in out-of-hospital cardiac arrest Next Article
Sacubitril-valsartan in hospitalized HF patients could have benefits »
Table of Contents: ACC/WCC 2020
Featured articles
Heart Failure and Cardiomyopathies
Mavacamten shows promising results in non-obstructive hypertrophic cardiomyopathy
Vericiguat shows beneficial effects in a very high-risk HF population
No role for sodium nitrite in out-of-hospital cardiac arrest
Vascular Medicine and Thromboembolism
Rivaroxaban and aspirin effective and safe for PAD patients
TAILOR-PCI misses endpoint but still provides valuable insights
Edoxaban: alternative to warfarin after surgical aortic or mitral valve procedures?
Bleeding reduction post-TAVI with OAC alone vs OAC + clopidogrel
Apixaban offers new perspective for cancer patients in need of anticoagulation
Rivaroxaban superior to enoxaparin in preventing VTE in non-major orthopaedic surgery
Interventional Cardiology
TAVR safe and effective in low-risk bicuspid aortic stenosis patients
TAVR model reveals differences in hospital outcomes
2-year results show non-significant outcomes TAVR vs surgery in severe aortic stenosis
Renal denervation better than sham for blood pressure
Infusion of ethanol in the vein of Marshall for persistent AF
Atrial Fibrillation/Acute Coronary Syndrome
Fewer adverse events with ticagrelor monotherapy after 3 months DAPT
TWILIGHT sub-study: same outcomes for diabetes patients
TWILIGHT sub-study: complex PCI patients
LAAO Watchman registry data positive
Apixaban in AF patients with recent ACS/PCI: Drop aspirin after 30 days
Genetics and Prevention
Homozygous FH responds to alirocumab
Evinacumab significantly reduces LDL-C in homozygous FH patients
Higher serum levels of eicosapentaenoic acid correlate with reduced CV events
Quit smoking: vaping + counselling helps
Related Articles

September 7, 2020
Letter from the Editor
September 8, 2020
LAAO Watchman registry data positive
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

