Dr James Freeman (Yale University School of Medicine, USA) presented the observational, non-randomised, post-approval analysis from the registry of periprocedural outcomes of the Watchman LAAO device, which includes data from >38,000 patients, [1]. The study was simultaneously published by the Journal of the American College of Cardiology [2].
The approval of LAAO to prevent stroke in patients with atrial fibrillation (AF) was established in 2015, based on 2 randomised trials, yet post-approval clinical data are limited [3,4]. To qualify for Medicare reimbursement in the USA, hospitals have been required to report all Watchman device procedures to the NCDR LAAO registry, with the intent to capture patient, hospital, and physician characteristics and in-hospital adverse event rates.
Compared with the original trials, the registry cohort was older (mean patient age was 76.1±8.1 years) and had more comorbidities (see Table). Furthermore, the mean CHA2DS2-VASc score for AF stroke risk determination was 4.6±1.5 (approximately 10-12% risk of stroke), and the mean HAS-BLED score for major bleeding was 3.0±1.1.
Table: Patient characteristics LAAO registry compared with other trials [1]
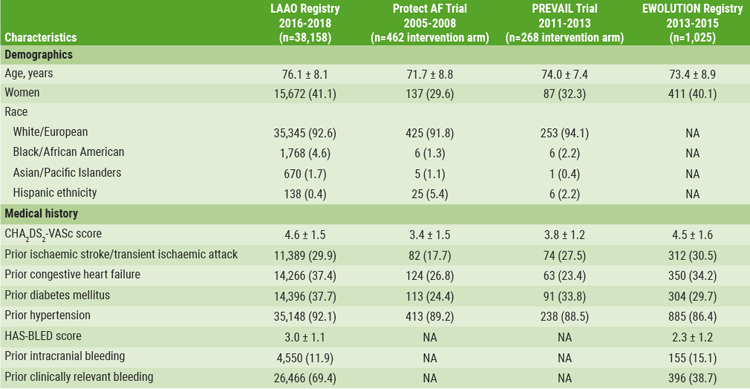
Hospitals performed a median number of 30 annual LAAO procedures, with a median of 12 per physician. Procedures were cancelled or aborted in 7% of cases; among cases in which a device was deployed, 98.1% were implanted with <5 mm leak. Major in-hospital adverse event rates were lower when compared to the original trials, occurring in 2.16% of patients, with the most common complications being pericardial effusion requiring intervention (1.39%) and major bleeding (1.25%), while stroke (0.17%) and death (0.19%) were rare.
The registry suggests that this procedure and device are safe and effective in managing stroke risk in a real-world setting with heterogeneous patient characteristics and comorbidities. However, without a control group, Dr Freeman cautioned, unmeasured confounders could influence the interpretation. For example, the adverse events reporting relies on site-reported data, which may be under-reported. Consequently, adverse events may be underestimated in this registry. However, overall, these registry data support the clinical trial data.
- Freeman JV, et al. Abstract 409-10. ACC/WCC 28-30 March 2020.
- Freeman JV, et al. J Am Coll Cardiol. 2020;75(13):1503-1518.
- Holmes DR Jr, et al. [Published correction appears in J Am Coll Cardiol. 2014 Sep 16;64(11):1186]. J Am Coll Cardiol. 2014;64(1):1–12.
- Reddy VY, et al. Circulation. 2013;127(6):720–729.
Posted on
Previous Article
« COVID-19 not associated with heightened VTE risk after discharge Next Article
Therapeutic anticoagulation not associated with lower mortality rates in COVID-19 ICU patients »
« COVID-19 not associated with heightened VTE risk after discharge Next Article
Therapeutic anticoagulation not associated with lower mortality rates in COVID-19 ICU patients »
Table of Contents: ACC/WCC 2020
Featured articles
Heart Failure and Cardiomyopathies
Mavacamten shows promising results in non-obstructive hypertrophic cardiomyopathy
Vericiguat shows beneficial effects in a very high-risk HF population
No role for sodium nitrite in out-of-hospital cardiac arrest
Vascular Medicine and Thromboembolism
Rivaroxaban and aspirin effective and safe for PAD patients
TAILOR-PCI misses endpoint but still provides valuable insights
Edoxaban: alternative to warfarin after surgical aortic or mitral valve procedures?
Bleeding reduction post-TAVI with OAC alone vs OAC + clopidogrel
Apixaban offers new perspective for cancer patients in need of anticoagulation
Rivaroxaban superior to enoxaparin in preventing VTE in non-major orthopaedic surgery
Interventional Cardiology
TAVR safe and effective in low-risk bicuspid aortic stenosis patients
TAVR model reveals differences in hospital outcomes
2-year results show non-significant outcomes TAVR vs surgery in severe aortic stenosis
Renal denervation better than sham for blood pressure
Infusion of ethanol in the vein of Marshall for persistent AF
Atrial Fibrillation/Acute Coronary Syndrome
Fewer adverse events with ticagrelor monotherapy after 3 months DAPT
TWILIGHT sub-study: same outcomes for diabetes patients
TWILIGHT sub-study: complex PCI patients
LAAO Watchman registry data positive
Apixaban in AF patients with recent ACS/PCI: Drop aspirin after 30 days
Genetics and Prevention
Homozygous FH responds to alirocumab
Evinacumab significantly reduces LDL-C in homozygous FH patients
Higher serum levels of eicosapentaenoic acid correlate with reduced CV events
Quit smoking: vaping + counselling helps
Related Articles
September 8, 2020
TAVR safe and effective in low-risk bicuspid aortic stenosis patients
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

