The multicentre, randomised, double-blind VOYAGER PAD trial was presented by Prof. Marc Bonaca (University of Colorado, USA), and simultaneously published in the New England Journal of Medicine [2]. The purpose of this trial was to investigate whether the benefits of a treatment strategy of rivaroxaban added to background antiplatelet therapy, which has been shown to reduce ischaemic risk in patients following recent acute coronary syndromes, could extend to patients with symptomatic PAD who had recently undergone lower limb revascularisation.
The trial enrolled 6,564 participants from 34 countries with moderate-to-severe symptomatic PAD in the lower extremities. Of these participants, 65% underwent endovascular revascularisation within 10 days before starting study treatment. The remaining 35% had undergone surgical revascularisation. Patients received either rivaroxaban 2.5 mg twice daily added to low-dose aspirin (n=3,286) or placebo + aspirin (n=3,278). The primary endpoint was a composite of acute myocardial infarction, ischaemic stroke, cardiovascular death, acute limb ischaemia, or major amputation. Median follow-up was 28 months.
The primary endpoint occurred in 508 patients in the rivaroxaban group, compared with 584 patients in the placebo group. The Kaplan-Meier estimates of incidence at 3 years were 17.3% of patients who received rivaroxaban + aspirin and 19.9% of those on placebo + aspirin (HR 0.85; 95% CI 0.76-0.96; P=0.0085; see Figure), translating to a 15% relative risk reduction in the rivaroxaban arm. The absolute risk reduction was 1.5% at 6 months, 2.0% at 1 year, and 2.6% at 3 years. This endpoint was primarily driven by a reduction in acute limb ischaemia.
Figure: Cumulative incidence of the primary endpoint versus time [1]
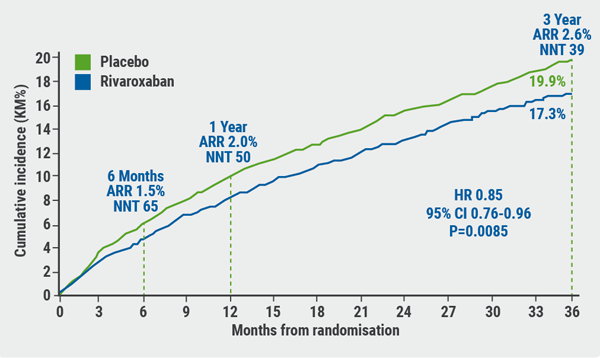
ARR, absolute risk reduction; CI, confidence interval; HR, hazard ratio; NNT, number needed to treat. Figure kindly provided by Prof. Bonaca.
Regarding safety, the rate of TIMI major bleeds was 2.7% for patients treated with rivaroxaban versus 1.9% for patients on placebo (HR 1.43; 95% CI 0.97-2.10; P=0.07). Although this result did not reach statistical significance, it did demonstrate a small but important increased risk for bleeding events of this combined regimen, which was underscored by the secondary safety outcome, ISTH major bleeding score, occurring in 5.9% and 4.1% of the rivaroxaban and placebo groups, respectively (HR 1.42; 95% CI 1.10-1.84; P=0.007). Despite bleeding events being more common in the patients receiving rivaroxaban, the rate of ischaemic events prevented by rivaroxaban + aspirin exceeded the excess bleeding rate by 3- to 6-fold (depending on the definition of the bleeding episodes).
In conclusion, PAD patients who have undergone lower-extremity revascularisation and take rivaroxaban plus aspirin have a lower incidence of major adverse limb and cardiovascular events than patients who take aspirin alone in this population and setting. In addition, the benefit of rivaroxaban was apparent early in treatment and was consistent over time across all major subgroups, including patients with critical limb-threatening ischaemia, and reduced the need for unplanned index limb revascularisation.
- Bonaca MP, et al. Abstract 402-10. ACC/WCC 28-30 March 2020.
- Bonaca MP, et al. N Engl J Med. 2020; DOI: 10.1056/NEJMoa2000052.
Posted on
Previous Article
« Subgroup analysis VOYAGER PAD Next Article
Letter from the Editor »
« Subgroup analysis VOYAGER PAD Next Article
Letter from the Editor »
Table of Contents: ACC/WCC 2020
Featured articles
Heart Failure and Cardiomyopathies
Mavacamten shows promising results in non-obstructive hypertrophic cardiomyopathy
Vericiguat shows beneficial effects in a very high-risk HF population
No role for sodium nitrite in out-of-hospital cardiac arrest
Vascular Medicine and Thromboembolism
Rivaroxaban and aspirin effective and safe for PAD patients
TAILOR-PCI misses endpoint but still provides valuable insights
Edoxaban: alternative to warfarin after surgical aortic or mitral valve procedures?
Bleeding reduction post-TAVI with OAC alone vs OAC + clopidogrel
Apixaban offers new perspective for cancer patients in need of anticoagulation
Rivaroxaban superior to enoxaparin in preventing VTE in non-major orthopaedic surgery
Interventional Cardiology
TAVR safe and effective in low-risk bicuspid aortic stenosis patients
TAVR model reveals differences in hospital outcomes
2-year results show non-significant outcomes TAVR vs surgery in severe aortic stenosis
Renal denervation better than sham for blood pressure
Infusion of ethanol in the vein of Marshall for persistent AF
Atrial Fibrillation/Acute Coronary Syndrome
Fewer adverse events with ticagrelor monotherapy after 3 months DAPT
TWILIGHT sub-study: same outcomes for diabetes patients
TWILIGHT sub-study: complex PCI patients
LAAO Watchman registry data positive
Apixaban in AF patients with recent ACS/PCI: Drop aspirin after 30 days
Genetics and Prevention
Homozygous FH responds to alirocumab
Evinacumab significantly reduces LDL-C in homozygous FH patients
Higher serum levels of eicosapentaenoic acid correlate with reduced CV events
Quit smoking: vaping + counselling helps
Related Articles

September 5, 2020
ACC.20/WCC Highlights Podcast Part 2 of 3
September 7, 2020
Vericiguat shows beneficial effects in a very high-risk HF population
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

