Patients with COVID-19 frequently experience venous and arterial thromboembolism. Elevated levels of D-dimer, a thrombotic biomarker, are harbingers of poorer clinical outcomes (i.e. disease progression and mortality). Data is needed regarding the type, dosage, and duration of an anticoagulation strategy.
Prof. Renato Lopes (Duke University Medical Center, NC, USA) presented the results of the Coalition ACTION trial (NCT04394377), which was designed to investigate the effectiveness of a full anticoagulation strategy compared with a prophylactic anticoagulation strategy in patients who had been hospitalised for COVID-19 and had elevated D-dimer levels. One group (n=311) received a 30-day course of rivaroxaban (20 mg once daily); the comparison group (n=304) received the standard in-hospital prophylactic venous thromboembolism protocol. The primary outcome was a hierarchical analysis of mortality, length of hospital stay, and duration of oxygen therapy over the 30-day intervention period. Results were analysed using an unmatched win ratio method, stratified by clinical severity.
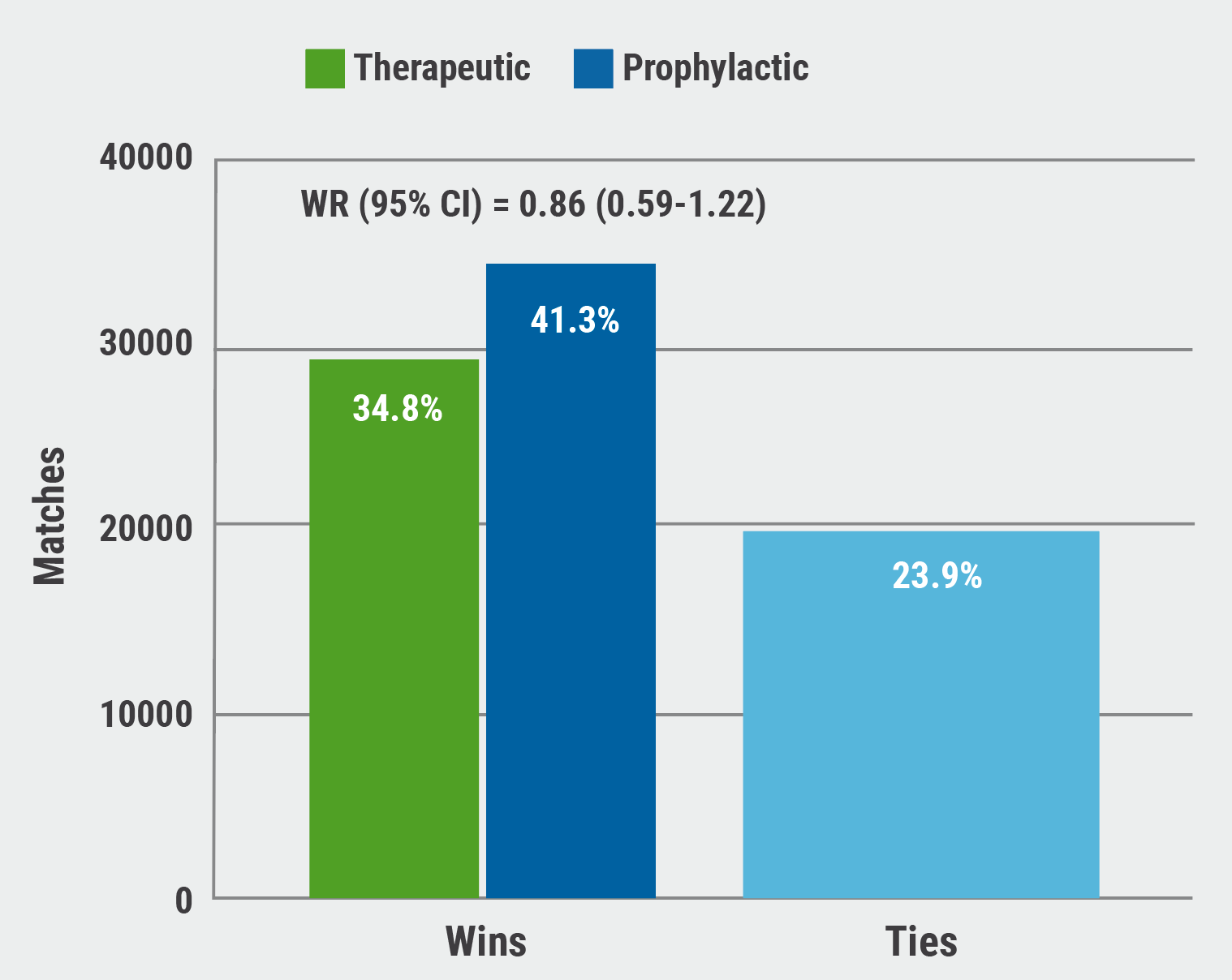
At 30 days, analysis of primary outcome data demonstrated that 41.3% of participants in the prophylactic group had won compared with only 34.8% of participants in the rivaroxaban group. The remaining 23.9% were tied (see Figure).
Figure: Primary outcome results at 30 days in the COALITION ACTION trial [1]
The primary safety outcome of the study was a major or clinically relevant non-major bleed, as designated by the International Society on Thrombosis and Haemostasis criteria. In the rivaroxaban group, 26 bleeds occurred (8.4%) versus 7 bleeds in the prophylactic group (2.3%), yielding a relative risk ratio of 3.64% (95% CI 1.61–8.27). In terms of all-cause mortality, 35/310 patients (11.3%) in the rivaroxaban group died compared with 23/304 patients in the prophylactic anticoagulation group (7.6%), yielding a relative risk ratio of 1.49 (95% CI 0.90–2.46).
The investigators concluded that therapeutic anticoagulation with rivaroxaban in patients hospitalised for COVID-19 did not improve clinical outcomes and increased bleeding when compared with in-hospital prophylactic anticoagulation.
- Lopes RD. Randomised Clinical Trial to Evaluate A Routine Full Anticoagulation Strategy in Patients with Coronavirus Infection (SARS-CoV-2) Admitted to Hospital: The Coalition ACTION Trial. Abstract 409-14, ACC 2021 Scientific Session, 15–17 May.
- Lopes RD, et al. Lancet 2021;397(10291):2253-63.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Older adults with heart failure benefit from rehabilitation programme Next Article
Rivaroxaban reduces total ischaemic events after peripheral artery revascularisation »
« Older adults with heart failure benefit from rehabilitation programme Next Article
Rivaroxaban reduces total ischaemic events after peripheral artery revascularisation »
Table of Contents: ACC 2021
Featured articles
Electrophysiology
Favourable outcomes with transcatheter atrial appendage occlusion
Etripamil nasal spray significantly improves PSVT-related symptoms
Ablation-based rhythm control as effective as rate control in AF and HF
Finerenone reduces the risk of AF onset in patients with CKD and diabetes
Heart Failure and Cardiomyopathy
PARADISE-MI: Sacubitril/valsartan not superior to ramipril in reducing HF events
Older adults with heart failure benefit from rehabilitation programme
Quality improvement intervention fails to improve care for patients with heart failure
Sacubitril/valsartan does not reduce NT-proBNP versus valsartan alone in HFrEF
Novel use of ivabradine in reversible cardiomyopathy
Mavacamten significantly improves QoL of patients with hypertrophic cardiomyopathy
Interventional and Structural Cardiology
Men and women benefit equally from early aspirin withdrawal following PCI
Similar outcomes with fractional flow reserve and angiography-guided revascularisation
TALOS-AMI: Exploring outcomes after switching to clopidogrel versus ticagrelor at 1 month from MI
Clopidogrel monotherapy associated with better net outcomes relative to aspirin monotherapy 6-18 months after PCI
Ischaemic Heart Disease
No difference in ischaemic risk or bleeding with low vs high-dose aspirin for secondary prevention: Lessons and questions from the ADAPTABLE trial
Rivaroxaban reduces total ischaemic events after peripheral artery revascularisation
Moderate hypothermia not superior to mild hypothermia following out-of-hospital cardiac arrest
Better outcomes with invasive strategy if anatomic complete revascularisation is possible
Prevention and Health Promotion
STRENGTH trial fails to demonstrate cardioprotective effect of omega-3 fatty acids
Evinacumab lowers triglyceride levels in severe hypertriglyceridaemia
Health equity and the role of the cardiologist: 7 priorities to consider
COVID-19
Dapagliflozin fails to show a significant protective effect in COVID-19
Therapeutic anticoagulation not superior to prophylactic anticoagulation in COVID-19
Atorvastatin does not reduce mortality in COVID-19
Valvular Heart Disease
Apixaban outcomes similar to current standard of care following TAVR
Preliminary results encouraging for EVOQUE tricuspid valve replacement
Related Articles

June 4, 2021
ACC 2021 Highlights Podcast
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

