The risk of thrombosis is highest in the first 30 days following acute MI. Bleeding is also higher; however, the curve may be less steep depending on patient bleeding risk and experience with prior antithrombotics, (see Figure) [2]. Currently, patients who have experienced acute MI followed by PCI receive DAPT with aspirin and a P2Y12 inhibitor. It was unknown whether de-escalation to a less potent, more variable P2Y12 inhibitor following the 30-day post-PCI would be associated with less bleeding.
Figure: The risk of thrombosis versus bleeding following acute myocardial infarction [2]
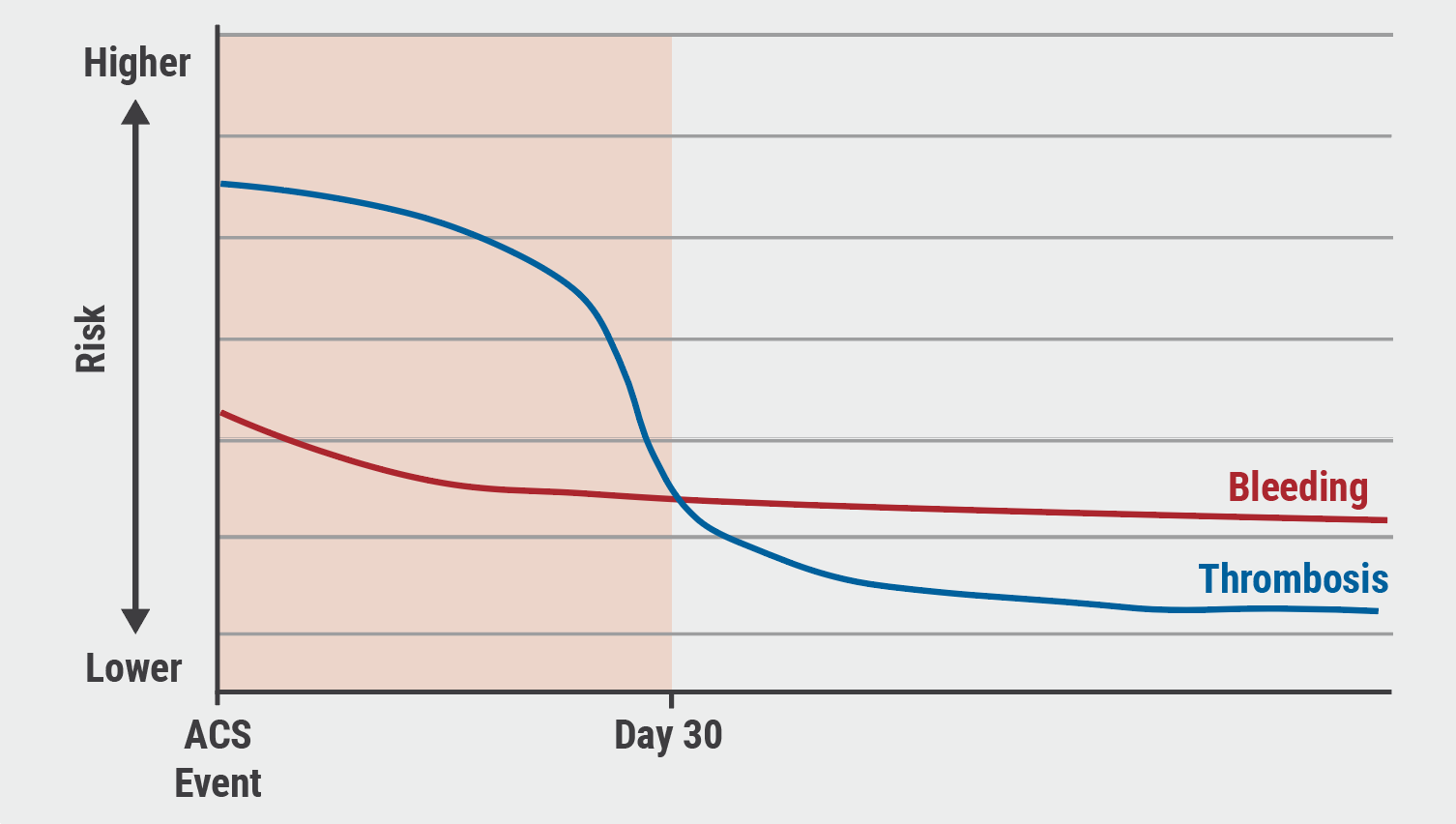
The TALOS-AMI trial (NCT02018055) aimed to address this question. The open-label study, presented by Prof. Kiyuk Chang (Seoul St. Mary’s Hospital, South Korea), compared outcomes of 2,679participants who, after 1 month of taking aspirin plus ticagrelor following acute MI and PCI, were randomised to clopidogrel (n=1,349) versus ticagrelor (n=1,348). Participants were monitored for 11 months (i.e. 1 year following MI and PCI).
The primary outcome was a composite of cardiovascular death, MI, stroke, or bleeding academic research consortium (BARC) bleeding type 2, 3, or 5 between 1 month and 12 months post PCI. At 1 year, 59 (4.6%) primary outcome events had occurred in the clopidogrel group compared with 104 (8.2%) events in the ticagrelor group, yielding a hazard ratio of 0.55 (95% CI 0.40–0.76; P<0.001 for both non-inferiority and superiority).
Prof. Chang and colleagues concluded that in patients who had experienced AMI and had no adverse events in the first month following PCI, a de-escalation DAPT strategy in selected patients switching from ticagrelor to clopidogrel was associated with lower net outcomes versus continued DAPT using ticagrelor. This observation was driven mostly by non-severe bleeding events, and power to distinguish a cost in terms of ischaemic outcome was limited.
The researchers acknowledged that this trial had significant limitations; it was open-label and not placebo-controlled. In addition, it was underpowered to detect a difference in ischaemic risk, especially in contrast to the PLATO trial, which randomised more than 18,000 patients and demonstrated superiority of ticagrelor versus clopidogrel in ACS [3]. Additionally, the study was conducted entirely in South Korea, and there is a high prevalence of CYP2C19 loss-of-function alleles in Koreans; for this reason, this de-escalation strategy should also be evaluated in a more heterogeneous population.
- Chang K. A Prospective, Multi-centre, Randomised, Open-label Trial to Compare Efficacy and Safety of Clopidogrel Versus Ticagrelor in Stabilized Patients with Acute Myocardial Infarction After Percutaneous Coronary Intervention. Abstract 407-10, ACC 2021 Scientific Session, 15–17 May.
- Rodriguez F, Harrington RA. N Engl J Med 2021 Feb 4;384(5):452–460.
- Wallentin L, et al.N Engl J Med 2009;361:1045-1057.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Prasugrel better than ticagrelor in ACS patients undergoing PCI Next Article
Quality improvement intervention fails to improve care for patients with heart failure »
« Prasugrel better than ticagrelor in ACS patients undergoing PCI Next Article
Quality improvement intervention fails to improve care for patients with heart failure »
Table of Contents: ACC 2021
Featured articles
Electrophysiology
Favourable outcomes with transcatheter atrial appendage occlusion
Etripamil nasal spray significantly improves PSVT-related symptoms
Ablation-based rhythm control as effective as rate control in AF and HF
Finerenone reduces the risk of AF onset in patients with CKD and diabetes
Heart Failure and Cardiomyopathy
PARADISE-MI: Sacubitril/valsartan not superior to ramipril in reducing HF events
Older adults with heart failure benefit from rehabilitation programme
Quality improvement intervention fails to improve care for patients with heart failure
Sacubitril/valsartan does not reduce NT-proBNP versus valsartan alone in HFrEF
Novel use of ivabradine in reversible cardiomyopathy
Mavacamten significantly improves QoL of patients with hypertrophic cardiomyopathy
Interventional and Structural Cardiology
Men and women benefit equally from early aspirin withdrawal following PCI
Similar outcomes with fractional flow reserve and angiography-guided revascularisation
TALOS-AMI: Exploring outcomes after switching to clopidogrel versus ticagrelor at 1 month from MI
Clopidogrel monotherapy associated with better net outcomes relative to aspirin monotherapy 6-18 months after PCI
Ischaemic Heart Disease
No difference in ischaemic risk or bleeding with low vs high-dose aspirin for secondary prevention: Lessons and questions from the ADAPTABLE trial
Rivaroxaban reduces total ischaemic events after peripheral artery revascularisation
Moderate hypothermia not superior to mild hypothermia following out-of-hospital cardiac arrest
Better outcomes with invasive strategy if anatomic complete revascularisation is possible
Prevention and Health Promotion
STRENGTH trial fails to demonstrate cardioprotective effect of omega-3 fatty acids
Evinacumab lowers triglyceride levels in severe hypertriglyceridaemia
Health equity and the role of the cardiologist: 7 priorities to consider
COVID-19
Dapagliflozin fails to show a significant protective effect in COVID-19
Therapeutic anticoagulation not superior to prophylactic anticoagulation in COVID-19
Atorvastatin does not reduce mortality in COVID-19
Valvular Heart Disease
Apixaban outcomes similar to current standard of care following TAVR
Preliminary results encouraging for EVOQUE tricuspid valve replacement
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

