After studying (new) drugs as monotherapy, in the last years multiple trials with combination therapy have been performed. Firstly, Prof. Raghu mentioned a trial comparing nintedanib with or without sildenafil in IPF patients, published on September 15th in the NEJM [9]. Sildenafil, a phosphodiesterase-5 inhibitor, causes predominantly pulmonary vasodilation. It also reduces endothelial cell apoptosis and vascular smooth muscle cell hypertrophy. In the STEP-IPF trial, published in 2010, in patients with IPF and DLCO <35% predicted, there was no significant difference between sildenafil and placebo on the primary endpoint, i.e. proportion of patients with improvement in 6MWD of ≥20% at week 12. Secondary endpoints of change in DLCO, dyspnoea, and SGRQ score were in favour of sildenafil [10]. The INSTAGE trial recently showed no significant difference in the adjusted mean change from baseline in the SGRQ (primary endpoint) total score at week 12 between the nintedanib plus sildenafil group and the nintedanib group (−1.28 points vs −0.77 points, respectively; P=0.72; see Figure). Also, no benefit from sildenafil treatment was observed with regard to dyspnoea, measured with the use of the Shortness of Breath Questionnaire. Decline in FVC was numerically lower in patients treated with nintedanib plus sildenafil vs nintedanib alone. Nintedanib plus sildenafil was associated with a reduction in risk of an absolute FVC decline of ≥5% predicted or death vs nintedanib alone. The change in FVC from baseline to 12 and 24 weeks in patients treated with nintedanib alone was -25.5 and -58.2 mL, respectively. This is comparable with the changes in FVC observed in the phase 3 INPULSIS trials.
Figure: INSTAGE: change from baseline in SGRQ total score at week 12 (primary endpoint) [9]
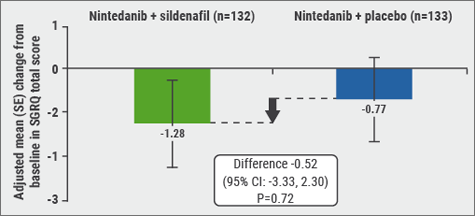
Diarrhoea was the most frequent AE. Nintedanib plus sildenafil had a manageable safety and tolerability profile, which was consistent with the profiles observed in patients with less advanced disease in the phase 2 TOMORROW trial and the phase 3 INPULSIS trial [11]. In INSTAGE, no new safety signals were identified with either treatment regimen in this very sick patient population. In conclusion, in patients with IPF and a DLCO of ≤35% predicted, (“a very sick patient population”, according to Prof. Raghu), nintedanib plus sildenafil did not provide a significant benefit as compared with nintedanib alone [9]. When interpreting these results, it should be taken into account that the participants had a severe impairment in gas exchanges (DLCO ≤35%). This group has largely been excluded from previous trials, and patients were treated for only 24 weeks. The INSTAGE data are important because in patients with severely impaired gas exchange, previously only limited data on the efficacy and safety of IPF treatments, including nintedanib, were available. These results seem to support the use of nintedanib across a range of IPF patients.
Long-term safety and tolerability of nintedanib
The efficacy and safety of nintedanib in IPF patients was assessed in two phase 3, placebo-controlled INPULSIS trials (n=807). Patients who completed the 52-week treatment period in an INPULSIS trial could receive open-label nintedanib in the extension trial, INPULSIS-ON (n=734, 91%). In INPULSIS, 59% of patients had received nintedanib and continued nintedanib in INPULSIS-ON. The remaining 41% had received placebo in and initiated nintedanib in INPULSIS-ON. The results from INPULSIS-ON were published in The Lancet Respiratory Medicine on September 14th [12]. In the INPULSIS-ON trial, the annual rate of decline in FVC over a median of almost 4 years was -135.1 mL/year. This was consistent with the annual rate of FVC decline in patients treated with nintedanib in the INPULSIS trials (-113.6 mL/year in patients treated with nintedanib). These data suggest that the effect of nintedanib on slowing disease progression of IPF persists beyond 4 years. The incidence rate of acute exacerbations in INPULSIS-ON was similar to that of INPULSIS. The safety profile of nintedanib in INPULSIS-ON was also consistent with that observed in INPULSIS. Diarrhoea was the most frequent AE in INPULSIS-ON, as was the case in the above mentioned INSTAGE trial. In total, 5% of patients who continued nintedanib and 10% of patients who initiated nintedanib permanently discontinued treatment because of diarrhoea. The AE that most frequently led to permanent discontinuation was progression of IPF (12% patients continuing nintedanib and 14% of patients initiating nintedanib) [12].
- Kolb M, et al. New Engl J Med. 2018, Sept 15.
- Zisman DA, et al. N Engl J Med. 2010;363:620-8.
- Richeldi L, et al. Respir Med. 2016;113:74-9.
- Crestani B, et al. Lancet Respir Med. 2018 Sep 14.
Posted on
Previous Article
« ICS: to use or not to use? Next Article
ALK inhibition, guidelines, liquid biopsies, and immunotherapy »
« ICS: to use or not to use? Next Article
ALK inhibition, guidelines, liquid biopsies, and immunotherapy »
Table of Contents: ERS 2018
Featured articles
Letter from The Editor
[Long Read] Current Look on Asthma
COPD: Triple Therapy, MABA and Antibiotics
Landmark triple therapy trials
ICS: to use or not to use?
MABA, and novel LAMA
Macrolide antibiotics and trial with azithromycin
Current Look on Asthma
[Long Read] Current Look on Asthma
Endoscopic Solutions
Endoscopic treatment of emphysema
Endoscopic treatment of asthma
Endoscopic treatment of chronic bronchitis
PAH
Balloon pulmonary angioplasty for CTEPH
New therapeutic targets: moving form pre-clinical data to phase 2 studies
IPF
Gastroesophageal reflux, IPF and lessons learned
Oncology
ALK inhibition, guidelines, liquid biopsies, and immunotherapy
Brain metastases, lung cancer and interstitial lung disease
Related Articles
November 7, 2018
IPF introduction and Pentraxin-2
November 7, 2018
EGFR-targeted treatments
November 7, 2018
Endoscopic treatment of asthma
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

