“The rationale behind targeting IL-1⍺ is that Th1 immunity plays a signifying role in adults with chronic AD,” said Prof. Alice Gottlieb (Icahn School of Medicine at Mount Sinai, USA). Under the influence of a variety of cytokines, including IL-17A and IL-17C, keratinocytes increase release of IL-1⍺. This cytokine drives leukocyte recruitment and activates the vascular endothelium [2]. By potentiation of nociceptors, itch is increased. It stimulates expression of matrix metalloproteinases, thus impairing the skin barrier [3]. This results in skin inflammation, epidermal barrier defects, and severe, debilitating itch, and these factor are all hallmarks of AD [4,5]. IL-1⍺ on circulating leukocytes may also drive inflammatory signalling in the microvasculature of lesions. In addition, it induces breakdown of the skin barrier by prompting matrix metalloproteinases. In animal studies, IL-1⍺ has shown to induce pruritus [6].
Therefore, an open-label, proof-of-concept, multicentre study evaluated the safety and efficacy of bermekimab, a monoclonal antibody targeting IL-1⍺, in patients with moderate-to-severe AD [1]. Participants were treated with 200 mg bermekimab (n=10) or 400 mg bermekimab (n=28). Injections were given weekly for 4 or 8 weeks, respectively.
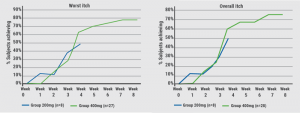
After 8 weeks, patients treated with 400 mg bermekimab achieved an improvement in the EASI by 75% from baseline, and a reduction in the Scoring of Atopic Dermatitis (SCORAD) by 67% (both differences P<0.0001). In addition, patients gained a significant better quality of life: 61% of patients in the high-dose group ended with a Dermatology Life Quality Index score of 0/1, which means that the disease no longer has an impact on their quality of life. Overall, 80% of patients treated with the higher dose achieved an improvement in pruritus (worst and average itch) scores on a Numeric Rating Scale of ≥ 4 points (see Figure). Significant improvements were also seen in anxiety and depression. At the beginning of the study, 71% of patients said they had a pain score of 7 out of 10. After 8 weeks, this was improved by more than 80%.
Figure: 80% of patients achieved a ≥4 points reduction in the worst and average itch NRS score [1]
There were 25 non-serious adverse events, which included no major adverse cardiac events and no neoplasms. Injection-site reactions were reported by 3%. The 400 mg dose provided greater efficacy without an increase in side effects. “The only side effects we noticed were grade 1 injection site reactions and nausea in 3 subjects. This surely is early data but it is still a nice study,” concluded Prof. Gottlieb.
- Gottlieb AB, et al. Late-breaking abstract D3T01.1F, EADV 2019, 9-13 Oct, Madrid, Spain.
- Guttman-Yassky E, Krüger JG. J Invest Dermatol 2018;138:1467-69.
- Guttman-Yassky E, Krüger JG. Curr Opin Immunol 2017;48:68-73.
- Gittler JK, et al. J Allergy Clin Immunol 2013;131:300-13.
- Yarbrough KB, et al. Dermatol Ther 2013;26:110-9.
- Katayama I, et al. J Invest Dermatol Symp Proc 2001;6:81-6.
Posted on
Previous Article
« IL-4/IL-13 blockade leads to rapid itch reduction in adolescents Next Article
Rituximab beats mycophenolate mofetil in pemphigus vulgaris »
« IL-4/IL-13 blockade leads to rapid itch reduction in adolescents Next Article
Rituximab beats mycophenolate mofetil in pemphigus vulgaris »
Table of Contents: EADV 2019
Featured articles
Late-Breaking News
IL-17A blocker effective in paediatric psoriasis patients
Rituximab beats mycophenolate mofetil in pemphigus vulgaris
Acne highly influenced by climate, pollutants, and unhealthy diet
JAK inhibition plus TCS lead to high clearance rates in AD
No cancer risk with long-term use of tacrolimus, a topical calcineurin inhibitor, in children with AD
Green light for a second JAK inhibitor in AD
Topical ruxolitinib effective in vitiligo
Emerging Therapies
Small molecules: interesting novel treatment options in AD
IL-1⍺ blockade: a new treatment option in AD
IL-4/IL-13 blockade leads to rapid itch reduction in adolescents
How to manage conjunctivitis in AD patients treated with a biologic
Biologics: increasingly used in paediatric dermatology
Spotlight on Psoriasis
IL-17 blocker: effective and safe in patients with comorbidities
ESPRIT registry: sharp decline in mortality in patients treated with a TNF blocker
Relationship psoriasis and NAFLD: new data on the hepato-dermal axis
Novel selective IL-23 blocker equally effective in patients with metabolic syndrome
Selective IL-23 blocker crushes fumaric acids in all assessed efficacy endpoints
No hint of teratogenicity through ixekizumab
New Insights in Photoprotection
Systemic photoprotection: a valuable addition to topical sun protection
The underestimated effect of visible light
Urticaria
Comorbidities more common in chronic urticaria, psoriasis, and AD
D-Dimer as future biomarker in CSU management?
Ligelizumab for CSU: symptom control and high response rates in re-treatment
Rosacea – From New Spectrum to New Therapy
New guidance on rosacea therapy according to phenotype
Best of the Posters
Above-the-neck melanoma more prone to metastases
Reduced sleep quality in dermatoses influenced by itch and pain
Anxiety and depression are common in families of AD infants
Certolizumab pegol efficacious for head and neck psoriasis
Related Articles
November 5, 2022
Novel oral psoriasis drug maintains efficacy over 2 years

© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

