Participants are 20 subjects 45-78 years of age, with OFF state Hoehn and Yahr classification ≤3. Each dose cohort consists of 5 study subjects that received 1 of 4 doses of mesenchymal stem cells: 1, 3, 6, or 10 x106 mesenchymal stem cells/kg of body weight. The primary outcome is safety, defined as the absence of transfusion reactions, adverse events or organ damage.
There were no adverse reactions in the first 24 hours. In subsequent follow-up, the most common side effects were hypertension, arthralgia, and nausea (27% for each), mild and transient in all cases. The first 14 patients sustained a reduction in Unified Parkinson Disease Rating Scale (UPDRS)-III motor score (OFF state) after 12 weeks. The researchers will complete the study to identify the optimal dose that is well-tolerated and associated with improvement in cognition, motor function, and disability.
1. Schiess M, et al. AAN 2019, S16.008.
Posted on
Previous Article
« First-ever effective and safe treatment of CMT1A Next Article
Eptinezumab reduces mean monthly migraine days »
« First-ever effective and safe treatment of CMT1A Next Article
Eptinezumab reduces mean monthly migraine days »
Table of Contents: AAN 2019
Featured articles
Letter from the Editor
Interview with Prof. Natalia Rost
Alzheimer's Disease and other Dementias
Amyloid PET in cognitively impaired patients
Tight blood pressure control lowers risk of mild cognitive impairment
Epilepsy
Headache and Migraine
Multiple Sclerosis and NMOSD
Immune tolerance by peptide-loaded tolerogenic dendritic cells
Biotin, ocrelizumab, and ibudilast in progressive MS
No increased MS relapse risk postpartum
Neuromuscular Disorders
First-ever effective and safe treatment of CMT1A
Parkinson’s Disease and other Movement Disorders
Leukaemia and hypertension therapies tested in Parkinson’s disease
Stroke
Miscellaneous
Possibly lifesaving therapy in refractory PML
New AAN guideline for treating Tourette syndrome
Subspecialty teleneurology: feasible and highly valued
Related Articles
July 30, 2019
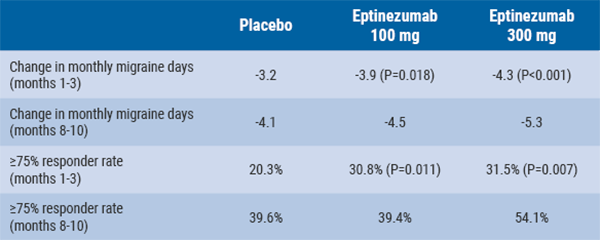
Eptinezumab reduces mean monthly migraine days
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

